Karyotypic analysis of Crucian carp, Carassius carassius (Linnaeus, 1758) from cold waters of Kashmir Himalayas
DOI:
https://doi.org/10.36253/caryologia-2112Keywords:
Carassius carassius, Colchicineoptimization, metaphase chromosomekidney, hypotonic solutionAbstract
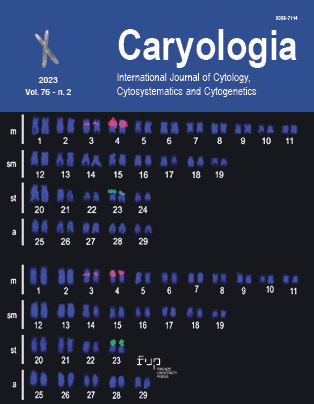
Carassius carassius (Linnaeus, 1758) is an exotic fish to Kashmir, locally known as “gang gad” and commonly called as “crucian carp”. It belongs to family Cyprinidae. The present study aimed to identify the chromosome number of the Carassius carassius and to optimize the colchicine concentrations (0.01%, 0.025%, and 0.05%) and hypotonic treatment timings (25, 35, and 45 minutes) for the chromosome preparation in Carassius carassius in order to obtain the highest number of clear and identifiable metaphasic chromosomal spreads. Data collected was analyzed and the means of each treatment was compared. The findings of the present study indicated that there was a significant influence of colchicine concentration, hypotonic timings as well as colchicine concentration× hypotonic timings (P<0.01) on the number of metaphase chromosome spreads. Furthermore a significant (P<0.01) strong positive correlation obtained between colchicine concentrations, hypotonic timings and the number of metaphase chromosome spreads. The findings of the present study recommends further research into chromosomal modification techniques such as fish polyploid production, gynogenesis, androgenesis, and inter or intra-species hybridization is needed to generate unique and good inbred lines in aquaculture.
Downloads
References
Abdoli, A, 1999. The Inland water fishes of Iran. Natural and wildlife museum of Iran, Tehran pp. 378.
Al-Sabti, K, 1991. Handbook of genotoxic effects and fish chromosomes. Slovenia pp. 221
Arai, R, 1982. A Chromosome study on two cyprinid fishes, Acrossocheilus labiatus and Pseudorasbora pumila pumila, with notes on Eurasian cyprinids and their karyotypes. Bull. Natl. Sci. Mus. Tokyo pp. 131-152.
Armstrong S J, Jones G H, 2003. Meiotic cytology and chromosome behaviour in wild-type Arabidopsis thaliana. Journal of experimental botany, 54(380): 1-10.
Baksi S M, Means J C, 1988. Preparation of chromosomes from early stages of fish for cytogenetic analysis. Journal of fish biology, 32(3): 321-325
Barat, A, Sahoo, P K and Ponniah, A G, 2002. Karyotype and Nucleolar Organizer Regions (NORs) in a few hill Stream fishes. The Fifth Indian Fisheries Forum.
Bayat, D F and Woznicki, P. 2006. Verification of ploidy level in sturgeon larvae. Aquaculture Research 37: 1671-1675.
Bazaz, A I, Ahmad, I, Shah, T H, Arab, N U, (2022). Karyomorphometric analysis of fresh water fish species of India, with special reference to cold water fishes of Kashmir Himalayas. A Mini Review. Caryologia 75(1): 109-121. doi: 10.36253/caryologia-1362.
Boron, A, Kirtiklis, L, Porycka, K, Abe, S, Juchno, D, Grabowska, A, Duchnowska, K, Karolewska, M, Kuczewska, A, Miroslawska, U and Wierzibicki, P, 2010. Comparative cytogenetic analysis of two Carassius species (Pisces, Cyprinidae) using chromosome banding and FISH with rDNA. Chromosome Research 20(10): 749.
Calado, L L, Bertollo, L A C, Costa, G W W Fd, Molina, W F, 2013. Cytogenetic studies of Atlantic mojarras (Perciformes–Gerreidae): chromosomal mapping of 5S and 18S ribosomal genes using double FISH. Aquac Res, 44: 829–835
Caperta A, Delgado M, Ressurreic F, Meister A, Jones R, Viegas W, Houben A. 2006. Colchicine-induced polyploidization depends on tubulin polymerization in c-metaphase cells. Protoplasma, 227:147–153
Chiarelli, B, Ferrantelli, O, Cucchi, C, 1969. The caryotype of some teleostean fish obtained by tissues culture in vitro. Experientia 25(4): 426–427.
Chourrout, D. and Happe, A, 1986. Improved methods of direct chromosome preparation in rainbow trout, Salmo gairdneri. Aquaculture 52: 255–261
Clark, J W and Donoghue, P C J, 2018. Whole-genome duplication and plant macroevolution. Trends Plant Sci. 2(10): 933–945.
Denton, T E and Howell, W M, 1969. A technique for obtaining chromosomes from the scale epithelium of teleost fishes. Copeia 2: 392-393.
Fan, Z and Fox, D P, 1990. A new method for fish chromosome preparation. Journal of fish biology 37(4): 553-561.
Guerra, M. 2008. Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenet Genome Res. 120(3–4): 339–350
Hafez, R, Labat, R and Quiller, R, 1978. Etude cytogenetique chez quelques especes de cyprinides de la region Midi-Pyrenees. Bulletin de la Societe d’Histoire Naturelle de Toulouse 114(1–2): 122–159.
Hanfling B, Bolton P, Harley M, Carvalho G.R. (2005). A molecular approach to detect hybridisation between crucian carp (Carassius carassius) and non-indigenous carp species (Carassius spp. and Cyprinus carpio). Freshwater Biology 50(3): 403-417. doi: 10.1111/j.1365-2427.2004.01330.x
Henegariu O, Heerema N A, Wright L L, Brayward P, Ward D C, Vance G H, 2001. Improvements in cytogenetic slide preparation: Controlled chromosome spreading, chemical aging and gradual denaturing. Cytometry, 43: 101-109.
Holopainen, K J and Hyvarinain, H, 1985. Ecology and physiology of crucian carp Carassius carassius (L) in small conditions in fin fish ponds with anoxic conditions in winter. Verh. Internal. Verem. Limnol. 22(4): 2566-2570.
Ida H, Murofush I M, Fujiwara S, Fujino K, 1978. Preperation of Fish chromosomes by in vitro Cochicine treatment. Japanese Journal of Ichthyology, 24 (4): 281-284.
Kalbassi, M R, Hosseini, S V and Tahergorabi, R, 2008. Karyotype Analysis in Schizothorax zarudnyi from Hamoon Lake, Iran. Turkish Journal of Fisheries and Aquatic Sciences 8: 335-340.
Karami, A, Araghi, P E, Syed, M A, Wilson, S P, 2015. Chromosome preparation in fish: effects of fish species and larval age. International Aquatic Research, 7, 201-210
Kligerman A D, Bloom S E, 1977. Rapid chromosome preparations from solid tissues of fishes. Journal of fisheries research board of Canada, 34: 249-261
Knytl, M, Kalous, L and Rab, P, 2013. Karyotype and chromosome banding of endangered crucian carp, Carassius carassius (Linnaeus, 1758)(Teleostei, Cyprinidae). Comparative Cytogenetics 7(3): 205.
Knytl M, Fornaini NR, 2021. Measurement of Chromosomal Arms and FISH Reveal Complex Genome Architecture and Standardized Karyotype of Model Fish, Genus Carassius. Cells, 10(9):2343. https://doi.org/10.3390/cells10092343.
Kobayasi, H, Kawashima, Y and Takeuchi, N, 1970. Comparative chromosome studies in the genus Carassius, especially with a finding of polyploidy in the ginbuna (C. auratus langsdorfii) (Teleostei: Cyprinidae). Japanese Journal of Ichthyology 17: 153–160.
Kullander S O, Fang F, Delling B, Ahlander E, 1999.The fishes of the Kashmir Valley. River Jhelum, Kashmir Valley. Impacts on the Aquatic Environment: 99-168.
Lomax B, Tang S, Separovic E, Phillips D, Hillard E, Thomson T and Kalousek D K, 2000. Comparative genomic hybridization in combination with flow cytometry improves results of cytogenetic analysis of spontaneous abortions. The American Journal of Human Genetics, 66(5): 1516-1521.
Luca, C., Suciu, R., Costache, M, 2010. Comparative karyotype in different lineages of cyprinid fish (Teleostei: Cypriniformes: Cyprinidae). Studia Universitatis “Vasile Goldiş”, Seria Ştiinţele Vieţii; 20: 37-41.
Manna GK. 1983. Cytogenetic studies on fishes and Amphibia. Genetical Research in India. Indian Council of Agricultural Research Publication, pp. 886-898.
Mayr, B, Rab, P. and Kalat, M, 1986. NORs and counterstain-enhanced fluorescence studies in Cyprinidae of different ploidy level. Genetica 69(2): 111–118.
Mayr, E, 1982. Speciation and macroevolution. Evolution 36(6): 1119–1132
Moore C M, Best R G. 2001. Chromosomal genetic disease: structural aberrations. Encyclopedia of Life Sciences.
Okomoda VT, Koh IC, Hassan A, Amornsakun T, Moh JH, Shahreza SM. 2018. Optimization of the cytogenetic protocol for Pangasianodon hypophthalmus (Sauvage, 1878) and Clarias gariepinus (Burchell, 1822). Peer Journal, 6: e5712.
Pradeep P J, Srijaya T C, Zain R B M, Papini, A, Chatterji, A K, 2011. A simple technique for chromosome preparation from embryonic tissues of teleosts for ploidy verification. Caryologia, 64: 235–241.
Prokes M, Barus V. (1996). On the natural hybrid between common carp (Cyprinus carpio) and Prussian carp (Carassius auratus gibelio) in the Czech Republic. Folia Zoologica, 45: 277-282.
Rab, P and Collares-Pereira, M J, 1995. Chromosomes of european cyprinid fishes. Folia Zool. 44: 193-214
Raicu, P, Taisescu, E and Banarescu, P, 1981. Carassius carassius and C. auratus, a pair of diploid and tetraploid representative species (Pisces, Cyprinidae). Genetica 46: 233–240.
Rieder C L, Palazzo RE.1992. Colcemid and the mitotic cycle. J Cell Sci 102:387–392
Rishi, K K, 1989. Current status of fish cyto-genetics. Fish Genetics in India pp 728-756.
Roberts, F L, 1964. A chromosome study of twenty species of Centrachidae. Journal of Morphology 115 (3): 401-417.
Smartt, J. 2007. A possible genetic basis for species replacement: preliminary results of interspecific hybridisation between native crucian carp Carassius carassius (L.) and introduced goldfish Carassius auratus (L.). Aquatic Invasions, 2: 59-62. doi: 10.3391/ai.2007.2.1.7
Shafi, S, 2012. Study on fecundity and GSI of Carassius carassius from Dal Lake Kashmir. Journal of Biology, Agriculture and Healthcare 2(3): 68-75.
Shao C W, Wu, P F, Wang, X L, Tian, Y S, Chen, S L, 2010. Comparison of chromosome preparation methods for the different developmental stages of the half-smooth tongue sole, Cynoglossus semilaevis. Micron, 41:47–50
Sofradzija, A, Berberovic, L and Hadziselimovic, R, 1978. Hromosomske garniture karaša (Carassius carassius) i babuške (Carassius auratus gibelio)] Chromosome sets of Carassius carassius and Carassius auratus gibelio. Ichthyologia 10(1): 135–148.
Spoz, A, Boron, A, Porycka, K, Karolewska, M, Ito, D, Abe, S, Kirtiklis, L and Juchno, D, 2014. Molecular cytogenetic analysis of the crucian carp, Carassius carassius (Linnaeus, 1758) (Teleostei, Cyprinidae), using chromosome staining and fluorescence in situ hybridisation with rDNA probes. Comp Cytogenet 8: 233-248.
Taki, Y., Urvushido, T., Suzuki, A. and Seriziawa, C, 1977. A Comparative study of Puntius (Cyprinidae: Pisces). Proceedings of Japan Academy 53(B): 231-235.
Wouters J, Janson S, Lusková V, Olsén K.H. (2012). Molecular identification of hybrids of the invasive gibel carp Carassius auratus gibelio and crucian carp Carassius carassius in Swedish waters. Journal of Fish Biology 80(7): 2595-2604. doi: 10.1111/j.1095-8649.2012.03312.x
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Gousia jan, Azra Shah, Saima Andleeb, Duedana Qazi, Asim Iqbal Bazaaz, IRFAN AHMAD, Oyas Asimi, Anayitullah Chesti, Bilal A Bhat

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Copyright on any open access article in a journal published byCaryologia is retained by the author(s).
- Authors grant Caryologia a license to publish the article and identify itself as the original publisher.
- Authors also grant any third party the right to use the article freely as long as its integrity is maintained and its original authors, citation details and publisher are identified.
- The Creative Commons Attribution License 4.0 formalizes these and other terms and conditions of publishing articles.
- In accordance with our Open Data policy, the Creative Commons CC0 1.0 Public Domain Dedication waiver applies to all published data in Caryologia open access articles.