Synthesis, Structural Characterization, and Biological Evaluation of (E)-N-(4-Bromobenzylidene)-3-Methoxybenzohydrazide Monohydrate
Published 2024-03-04
Keywords
- Hybrid crystals,
- π···π interactions,
- Molecular docking,
- Hirshfeld surface,
- ADMET
How to Cite
Copyright (c) 2023 Kumar Ananthi, Haridhass Anandalakshmi, Amaladoss Nepolraj, Saravanan Akshaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
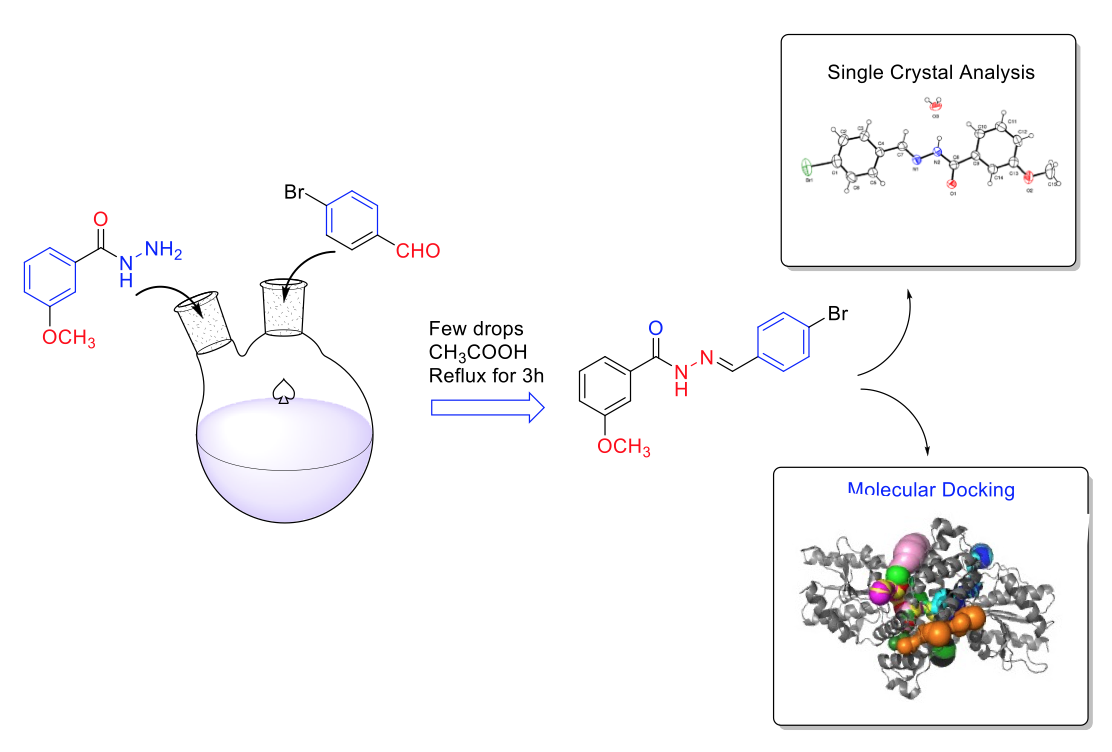
Synthesis and structural elucidation of a new type of hydrazone Schiff base (E)-N’-(4-Bromobenzylidene)-3-Methoxybenzohydrazide Monohydrate, and its structure were characterized by FT-IR, 1H, 13C NMR and mass spectroscopic analysis. The single crystals of (4-BRMBH) were grown from the DMSO solvent, orthorhombic system with P212121 space group through single-crystal X-ray diffraction analysis. DFT calculations were performed to understand the electronic properties including frontier molecular orbitals (FMO), molecular electrostatic potentials, and global chemical reactivity descriptors. Intermolecular interactions in the crystal structures were obtained using the Hirshfeld surface analysis. The majority contribution to the Hirshfeld surface is H···H (39.5%) contacts. The molecular docking study were carried out by in silico method to analyse their anti-tuberculosis aspect against InhA, the enoyl acyl carrier protein reductase from Mycobacterium tuberculosis. Finally, chemical absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties were determined.
References
- N.G. Kandile, M.I. Mohamed, H.M. Ismaeel, Synthesis of new Schiff bases bearing 1,2,4-triazole, thiazolidine and chloroazetidine moieties and their pharmacological evaluation, J. Enzyme Inhib. Med. Chem., 2017, 32, 119–129. https://doi.org/10.1080/14756366.2016.1238365
- U. Casellato, P.A. Vigato, M. Vidali, Transition metal complexes with binucleating ligands, Coord. Chem. Rev., 1977 23, 31–117. https://doi.org/10.1016/S0010-8545(00)80330-6
- K. Dey, A.K. Biswas, A. Roy, Metallic complexes as ligands: Part II-Nickcl(II) complex of the Schiff base derived from 3-formylsalicylic acid and ethylenediamine as ligand for Ti, Zr, Sn, P and B, Indian J. Chem., 1981, 20A, 848–851.
- L. Pogany, J. Moncol, M. Gal, I. Salitros, R. Boca, Four cobalt (III) Schiff base complexes – Structural, spectroscopic and electrochemical studies, Inorg. Chim. Acta., 2017 462, 23–29. https://doi.org/10.1016/j.ica.2017.03.001
- C. M. da Silva, D. L. da Silva, L. V. Modolo, R. B. Alves, M. A. Deresede, C. V. Martin, A. Defatima, Schiff bases: A short review of their antimicrobial activities, J. Adv. Res., 2011, 2, 1–8. https://doi.org/10.1016/j.jare.2010.05.004
- M. Andruh, Compartmental Schiff-base ligands – a rich library of tectons in designing magnetic and luminescent materials, Chem. Commun., 2011, 47, 3025–3042. https://doi.org/10.1039/C0CC04506C
- M. Sarigul, A. Sari, M. Kose, V. McKee, M. Elmastas, I. Demirtas, M. Kurtoglu, New bio-active azo-azomethine based Cu (II) complexes, Inorg. Chim. Acta., 2016, 444, 166– 175. https://doi.org/10.1016/j.ica.2016.01.042
- H. Keypour, M. Shayesteh, M. Rezaeivala, F. Chalabian, Y. Elerman, O. Buyukgungor, Synthesis, spectral characterization, structural investigation and antimicrobial studies of mononuclear Cu(II), Ni(II), Co(II), Zn(II) and Cd(II) complexes of a new potentially hexadentate N2O4 Schiff base ligand derived from salicylaldehyde, J. Mol. Struct., 2013 1032, 62–68. https://doi.org/10.1016/j.molstruc.2012.07.056
- D. Chen, A.E. Martell, Dioxygen affinities of synthetic cobalt Schiff base complexes, Inorg. Chem., 1987, 26, 1026–1030. https://doi.org/10.1021/ic00254a013
- D. Chen, A.E. Martell, Y. Sun, New synthetic cobalt Schiff base complexes as oxygen carriers, Inorg. Chem., 1989, 2, 2647–2652. https://doi.org/10.1021/ic00312a029
- K. Durka, A.A. Hoser, R. Kaminski, S. Lulinski, J. Serwatowski, W. Kozminski, K. Wozniak, Polymorphism of a model arylboronicazaester: Combined experimental and computational studies, Cryst. Growth. Des., 2011, 11, 1835–1845. https://doi.org/10.1021/cg200032e
- M. Behzad, L. SeifikarGhomi, M. Damercheli, B. Mehravi, M. ShafieeArdestani, H.SamariJahromi, Z. Abbasi, Crystal structures and in vitro anticancer studies on new unsymmetrical copper(II) Schiff base complexes derived from meso-1,2-diphenyl-1,2- ethylenediamine: a comparison with related symmetrical ones, J. Coord. Chem., 2016, 69, 2469–2481. https://doi.org/10.1080/00958972.2016.1198786
- Y. L. Zhang, W. J. Ruan, X. J. Zhao, H. G. Wang, Z. A. Zhu, Synthesis and characterization of axial coordination cobalt (III) complexes containing chiral Salen ligands, Polyhedron, 2003, 22, 1535–1545. https://doi.org/10.1016/S0277-5387(03)00261-4
- Z. Abbasi, M. Behzad, A. Ghaffari, H. AmiriRudbari, G. Bruno,Mononuclear and dinuclearsalen type copper(II) Schiff base complexes: Synthesis, characterization, crystal structures and catalytic epoxidation of cyclooctene, Inorg. Chim. Acta., 2014, 414, 78–84. https://doi.org/10.1016/j.ica.2014.01.047
- M. R.V. Jørgensen, I. Skovsen, H. F. Clausen, J. L. Mi, M. Christensen, E. Nishibori, M. A. Spackman, B. B. Iversen, Inorg. Chem., 2012, 51, 1916–1924. https://doi.org/10.1021/ic202231k
- Y. H. Luo, B. W. Sun, An investigation into the substituent effect of halogen atoms on the crystal structures of indole-3-carboxylic acid (ICA), CrystEngComm., 2013, 15, 7490–7497. https://doi.org/10.1039/C3CE40952J
- A. Ghaffari, M. Behzad, M. Pooyan, H. AmiriRudbari, G. Bruno, Crystal structures and catalytic performance of three new methoxy substituted salen type nickel (II) Schiff base complexes derived from meso-1,2-diphenyl-1,2-ethylenediamine, J. Mol. Struct., 2014, 1063, 1–7. https://doi.org/10.1016/j.molstruc.2014.01.052
- H. C. Wang, X. Q. Yan, T. L. Yan, H. X. Li, Z. C. Wang, Design, synthesis and biological evaluation of benzohydrazide derivatives containing dihydropyrazoles as potential EGFR kinase inhibitors, Molecules. 2016, 21, 1012. https://doi.org/10.3390/molecules21081012
- B. Rigo, D. Couturier, Studies on pyrrolidinones. Synthesis of 5-(5-oxo-2-pyrrolidinyl)-1,3,5-oxadiazole-2-thione derivatives, Heterocycl. Chem., 1985, 22, 287–288. https://doi.org/10.1002/jhet.5570220209
- M. Somashekhar, Synthesis and antimicrobial activity of 4-(morpholin-4-yl) benzohydrazide derivatives. World J. Pharm. Pharm. Sci., 2013, 2, 2011–2020.
- P. Nun, C. Martin, J. Martinez, F. Lamaty, Solvent-free synthesis of hydrazones and their subsequent N-alkylation in a Ball-mill, Tetrahedron, 2011, 67, 8187–8194. https://doi.org/10.1016/j.tet.2011.07.056
- P. Melnyk, V. Leroux, C. Sergheraert, P. Grellier, Bioorg. Med. Chem. Lett., 2006, 16, 31–35. https://doi.org/10.1016/j.bmcl.2005.09.058
- I. Afreen, M. Sonam, S. R. Maitreyi, A. Fernando, A. Amir, Synthesis and biological evaluation of 4-(2-(dimethylamino)ethoxy) benzohydrazide derivatives as inhibitors of Entamoebahistolyica, Eur. J. Med. Chem., 2016, 124, 445–455. https://doi.org/10.1016/j.ejmech.2016.08.022
- K. K. Bedia, O. Elcin, U. Seda, K. Fatma, S. Nathaly, Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity, Eur. J. Med. Chem., 2006, 41, 1253–1261. https://doi.org/10.1016/j.ejmech.2006.06.009
- H. Lgaz, I.-M. Chung, M. R. Albayati, A. Chaouiki, R. Salghi, S. K. Mohamed, Improved corrosion resistance of mild steel in acidic solution by hydrazone derivatives: An experimental and computational study, Arab. J. Chem., 2018, 2934–2954. https://doi.org/10.1016/j.arabjc.2018.08.004
- H. Lgaz, A. Chaouiki, M. R. Albayati, R. Salghi, Y. El Aoufir, I. H. Ali, M. I. Khan, S. K. Mohamed, I.-M. Chung, Synthesis and evaluation of some new hydrazones as corrosion inhibitors for mild steel in acidic media, Res. Chem. Intermed., 2019, 45, 2269–2286. https://doi.org/10.1007/s11164-018-03730-y
- Stoe and Cie, X-AREA (Version 1.18.) and X-RED32 (Version 1.04.), Stoe and Cie, Germany, Darmstadt, 2002.
- G.M. Sheldrick, SHELXT: Integrating space group determination and structure solution, ActaCrystallogr. 2015, A 71, 3–8. https://doi.org/10.1107/S2053273314026370
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek, P.A. Wood, Mercury CSD 2.0–new features for the visualization and investigation of crystal structures, J. Appl. Crystallogr. 2008, 41, 466–470. https://doi.org/10.1107/S0021889807067908
- L. J. Farrugia, WinGX suite for small-molecule single-crystal crystallography, J. Appl. Crystallogr. 1999, 32, 837–838. https://doi.org/10.1107/S0021889899006020
- S. P. Westrip, publCIF: software for editing, validating and formatting crystallographic information files, J. Appl. Crystallogr, 2010, 43, 920–925. https://doi.org/10.1107/S0021889810022120
- A. Spek, Single-crystal structure validation with the program PLATON, J. Appl. Crystallogr. 2003, 36, 7–13. https://doi.org/10.1107/S0021889802022112
- J. J. McKinnon, M. A. Spackman, A. S. Mitchell, Novel tools for visualizing and exploring intermolecular interactions in molecular crystals, J. ActaCrystallogr. 2004, B 60, 627–668. https://doi.org/10.1107/S0108768104020300
- M. A. Spackman, D. Jayatilaka, Hirshfeld surface analysis, CrystEngComm., 2009, 11, 19–32. https://doi.org/10.1039/B818330A
- M. Venkateshan, R. Vishnu Priya, M. Muthu, J. Suresh, R. Ranjith Kumar, Crystal structure, Hirshfeld surface analysis, DFT calculations and molecular docking studies on pyridine derivatives as potential inhibitors of NAMPT, Chem. Data Collect., 2019, 23, 100262. https://doi.org/10.1016/j.cdc.2019.100262
- C. Lee, W. Yang, R.G. Parr, Phys. Rev., 1988, B 37, 785–789. https://doi.org/10.1103/PhysRevB.37.785
- A.M. K€oster, M. Leboeuf, D.R. Salahub, in: S.M. Jane, S. Kalidas (Eds.), Molecular electrostatic potentials from density functional theory, Theor. Comput. Chem., 1996, 105–142. https://doi.org/10.1016/S1380-7323(96)80042-2
- M. A. Spackman, J. J. McKinnon, Fingerprinting intermolecular interactions in molecular crystals, CrystEngComm., 2002, 4, 378–392. https://doi.org/10.1039/B203191B
- S. Madan Kumar, B.C. Manjunath, G.S. Lingaraju, M.M.M. Abdoh, M.P. Sadashiva, N.K. Lokanath, A Hirshfeld surface analysis and crystal structure of 2-[1-(2-fluorophenyl)-1H-tetrazol-5-yl]-4-methoxybiphenyl-2-carbaldehyde, Cryst. Struct. Theor. Appl., 2013, 3, 124–131. DOI: 10.4236/csta.2013.23017
- A. Denise Rozwarski, C. Vilcheze, M. Sugantino, R. Bittman, J.C. Sacchettini, J. Biol. Chem., 1999, 274, 15582–15589. https://doi.org/10.1074/jbc.274.22.15582
- R. Maheswari, J. Manjula, Vibrational spectroscopic analysis and molecular docking studies of (E)-4-methoxy-N-(4-methylbenzylidene) benzohydrazide by DFT, J. Mol. Struct., 2016, 1115, 144–155. https://doi.org/10.1016/j.molstruc.2016.02.066
- M.I. Okeke, C.U. Iroegbu, E.N. Eze, A.S. Okoli, C.O. Esimone, Evaluation of extracts of the roots of Landolphiaowerrience for antibacterial activity, J. Ethnopharmacol., 2001, 78, 119–127. https://doi.org/10.1016/S0378-8741(01)00307-5
- A. Nepolraj, V. I. Shupeniuk, M, Sathiyaseelan N. Prakash. Synthesis of new 3‐(hydroxymethyl)‐2‐phenyl‐2, 3 dihydroquinolinone and in‐silico evaluation of COVID‐19 main protease inhibitor. Vietnam J Chemistry., 2021, 59, 511-521 https://doi.org/10.1002/vjch.202000221





