The puzzling problem of water properties at low temperature. An experimentalist view.
Published 2021-12-10
Keywords
- Water,
- Low temperature,
- Anomalies,
- Glass transition,
- Structural models
How to Cite
Copyright (c) 2019 José Teixeira

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
Water is at once the most familiar substance, the one for which we have the most data, and a liquid with anomalous properties that make it unique.
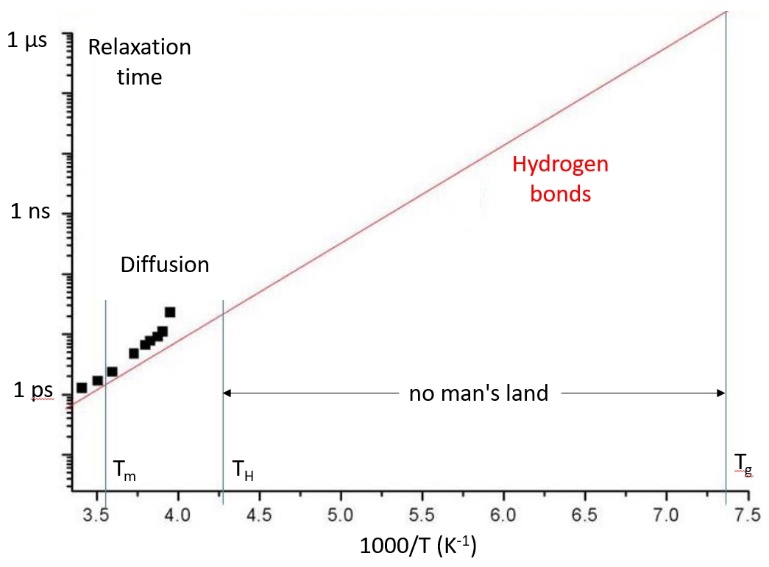
Many theoretical models provide explanations for the abnormal behaviour of water. The most recent ones are based on numerical simulations of molecular dynamics made from effective potentials that reproduce the tetrahedral geometry of hydrogen bonds. From the experimental side, homogeneous nucleation of hexagonal ice limits the range of temperature accessible to experiments. There is therefore no experimental data at atmospheric pressure in a wide temperature range extending from the homogeneous nucleation temperature (230 K) to the glass transition (135 K). However, water anomalies are the most important in the supercooled domain. Therefore, a large number of theoretical models, often based on extrapolations of data or analogies, have been developed without being able to be compared to non-existent experimental data. In all cases, the temperature domain where homogeneous nucleation takes place plays a crucial
role in the anomalies observed at low temperature. Here, we present shortly structural models that predict the existence of a low-temperature critical point and a liquid-liquid transition between two phases of different structures by comparing them with experimental data. Other models are based on dynamic transitions or the existence of two types of relaxation, at the molecular and hydrogen bonding levels, which may correspond to two glass transitions.




