Chemistry, Cyclophosphamide, Cancer Chemotherapy, and Serendipity: Sixty Years On
Published 2021-03-01
Keywords
- cyclophosphamide,
- cancer,
- metabolism,
- synthesis,
- stereochemistry
How to Cite
Copyright (c) 2020 Gerald Zon

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
Cambridge Dictionary: serendipity | noun | the phenomenon of finding interesting or valuable things by chance.
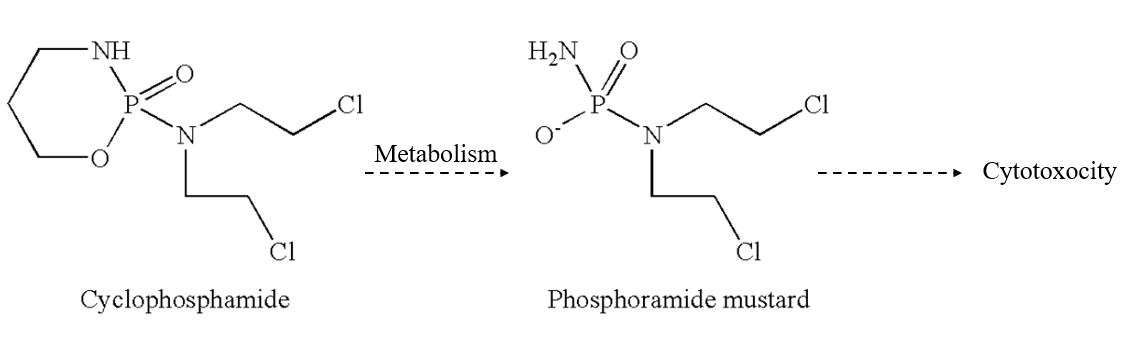
The year 2019 marked the 60th anniversary of the approval of cyclophosphamide (CP) as an anticancer by the U.S. Food & Drug Administration in 1959 for the treatment of lymphoma. Between 1959 and 2019 there were ~50,000 publications listed in PubMed that have CP in the title and/or abstract, with these annual numbers showing a continual increase, and over 1,800 such articles in 2019 alone. The discovery of CP is a prime example of serendipity in science, which also applies to key elements of the metabolism and pharmacological basis for the specificity of the cytotoxicity of CP toward cancer cells. Phosphoramide mustard (PM), HO(H2N)P(O)N(CH2CH2Cl)2, the principal metabolite of CP with DNA alkylating activity, was synthesized and reported by Friedman and Seligman in 1954 prior to the discovery of CP. Interestingly, the original drug design premise for synthesizing PM, which was based on elevated phosphamidase enzyme activity in cancer cells proved to be incorrect. While this wrong premise also led to the synthesis of CP, as a six-membered ring cyclic phosphamidase-activated precursor of PM, the actual metabolic conversion of CP to PM was subsequently found to involve a surprisingly complex array of metabolites and metabolic pathways, all completely unrelated to phosphamidase. Although the molecular structure of CP has an asymmetrically substituted, i.e. chiral phosphorus center, the racemic mixture of the Rp and Sp enantiomers of CP was used throughout its initial investigations and subsequent clinical trials despite the involvement of an initial enzyme-mediated metabolic activation step, which could, in principle, be stereoselective for only one of the enantiomers of CP. Stereochemical investigations along those lines were eventually carried out, but the results did not warrant replacement of racemic CP with either enantiomer in the clinic. Amazingly, there are now ~4,000 structural congeners of PM listed Chemical Abstracts, but none have led to an anticancer drug superior to CP. This account provides a synopsis of the key chemistry and stereochemistry investigations that comprise this story of CP, as a remarkable instance of serendipity in science, and my chance involvement in the unfolding of this fascinating story.
References
- N. Drake, Nature Medicine 2011, 17, 757-757.
- K. E. DeBruin, K. Naumann, G. Zon, K. Mislow, Journal of the American Chemical Society 1969, 91, 7031-7040.
- N. Brock, Cancer Res 1989, 49, 1-7.
- J. Conant, Smithsonian 2020, 51, 66-80.
- W. B. Morrison, J Veterinary Int Med 2010, 24, 1249-1262.
- O. M. Friedman, A. M. Seligman, J Amer Chem Soc 1954, 76, 655-658.
- A. M. Seligman, M. M. Nachlas, L. H. Manheimer, O. M. Friedman, G. Wolf, Ann Surgery 1949, 130, 333-341.
- A. M. Rutenburg, A. M. Seligman, L. Persky, O. M. Friedman, J Pharmacol Exp Therap 1954, 111, 483-488.
- H. Arnold, F. Bourseaux, N. Brock, Naturwissenschaften 1958, 45, 64-66.
- R. Gross, G. Wulf, Strahlentherapie 1959, 41, 361-367.
- P. R. Coggins, R. G. Ravdin, S. H. Eisman, Cancer 1960, 13, 1254-1260.
- M. Matthias, R. Sohr, R. Preiss, B. Brockmann, Onkologie 1984, 7, 48-49.
- J. Cohen, J. Jao, W. Jusko, British Journal of Pharmacology 1971, 43, 677.
- D. L. Hill, M. C. Kirk, R. F. Struck, J Amer Chem Soc 1970, 92, 3207-3208.
- J. E. Bakke, V. J. Feil, R. G. Zaylskie, J Agricultural Food Chem 1971, 19, 788-790.
- M. Colvin, C. A. Padgett, C. Fenselau, Cancer Res 1973, 33, 915-918.
- T. A. Connors, P. J. Cox, P. B. Farmer, A. B. Foster, M. Jarman, Biochem Pharmacol 1974, 23, 115-129.
- C. Fenselau, M. N. Kan, S. S. Rao, A. Myles, O. M. Friedman, M. Colvin, Cancer Res 1977, 37, 2538-2543.
- T. W. Engle, G. Zon, W. Egan, J Med Chem 1979, 22, 897-899.
- Z. B. Papanastassiou, R. J. Bruni, E. White, V. Levins, P. L. Levins, J Med Chem 1966, 9, 725-729.
- T. W. Engle, G. Zon, W. Egan, J Med Chem 1982, 25, 1347-1357.
- R. F. Struck, Cancer Treat. Rep 1976, 60, 317-319.
- T. A. Connors, P. J. Cox, P. B. Farmer, A. B. Foster, M. Jarman, J. K. Macleod, Biomedical mass spectrometry 1974, 1, 130-136.
- N. Brock, J. Stekar, J. Pohl, U. Niemeyer, G. Scheffler, Arzneimittel-forschung 1979, 29, 659-661.
- H. J. Hohorst, U. Draeger, G. Peter, G. Voelcker, Cancer treatment reports 1976, 60, 309-315.
- C. Fenselau, J. P. Lehman, A. Myles, J. Brandt, G. S. Yost, O. M. Friedman, O. M. Colvin, Drug Metabol Dispos 1982, 10, 636-640.
- D. W. Hutchinson, J. A. Miller, W. J. Stec, Organophosphorus Chemistry 1982, 13, 145 - 174. .
- B. E. Domeyer, N. E. Sladek, Biochem Pharmacol 1980, 29, 2903-2912.
- G. Zon, S. M. Ludeman, J. A. Brandt, V. L. Boyd, G. Ozkan, W. Egan, K. L. Shao, J Med Chem 1984, 27, 466-485.
- G. Völker, U. Dräger, G. Peter, H. J. Hohorst, Arzneimittelforschung 1974, 24, 1172-1176. .
- J. E. Low, R. F. Borch, N. E. Sladek, Cancer Res 1982, 42, 830-837. .
- R. F. Borch, K. M. Getman, J Med Chem 1984, 27, 485-490.
- V. L. Boyd, M. F. Summers, S. M. Ludeman, W. Egan, G. Zon, J. B. Regan, J Med Chem 1987, 30, 366-374.
- R. H. Knop, C.-W. Chen, J. B. Mitchell, A. Russo, S. McPherson, J. S. Cohen, Biochim Biophys Acta (BBA) - Molec Cell Res 1984, 804, 275-284.
- V. L. Boyd, J. D. Robbins, W. Egan, S. M. Ludeman, J Med Chem 1986, 29, 1206-1210.
- G. Voelcker, T. Wagner, H. J. Hohorst, Cancer treatment reports 1976, 60, 415-422.
- G. S. Payne, C. R. Pinkerton, E. Bouffet, M. O. Leach, Magnetic Resonance in Medicine 2000, 44, 180-184.
- H. A. Dirven, J. C. Venekamp, B. van Ommen, P. J. van Bladeren, Chem Biol Interact 1994, 93, 185-196.
- H. A. Dirven, B. van Ommen, P. J. van Bladeren, Cancer Res 1994, 54, 6215-6220.
- L. Sreerama, N. E. Sladek, Clinical Cancer Research 1997, 3, 1901-1914.
- M. Magni, S. Shammah, R. Schiró, W. Mellado, R. Dalla-Favera, A. M. Gianni, Blood 1996, 87, 1097-1103.
- G. Zon, Tetrahedron Lett 1975, 16, 3139-3142.
- P. Kinas, K. Pankiewicz, W. J. Stec, Bull. Acad. Pol. Sci. 1975, 23, 981-984.
- G. Zon, J. A. Brandt, W. Egan, J Natl Cancer Inst 1977, 58, 1117-1119.
- J. T. Karle, I. Karle, Acta Crystallographica 1966, 21, 849-859.
- I. L. Karle, J. M. Karle, W. Egan, G. Zon, J. A. Brandt, J Am Chem Soc 1977, 99, 4803-4807.
- D. Adamiak, W. Saenger, R. Kinas, W. Stec, Zeitschrift für Naturforschung C 1977, 32, 672-677.
- T. Fai-Po, J. A. Brandt, G. Zon, Biochem Pharmacol 1979, 28, 367-374.
- M. Jarman, R. A. Milsted, J. F. Smyth, R. W. Kinas, K. Pankiewicz, W. J. Stec, Cancer Res 1979, 39, 2762-2767.
- K. Misiura, A. Okruszek, K. Pankiewicz, W. J. Stec, Z. Czownicki, B. Utracka, Journal of Medicinal Chemistry 1983, 26, 674-679.
- M. Williams, I. Wainer, Current pharmaceutical design 1999, 5, 665-672.
- A. R. Ahmed, S. M. Hombal, Journal of the American Academy of Dermatology 1984, 11, 1115-1126.
- S. Copeland, Synthese 2019, 196, 2385-2406.
- S. Ganguly, U. Kumar, N. Gupta, A. Guleria, S. Majumdar, S. Phatak, S. Chaurasia, S. Kumar, A. Aggarwal, D. Kumar, R. Misra, Lupus 2020, 29, 782-786.
- G. E. Foley, O. M. Friedman, B. P. Drolet, Cancer Res 1961, 21, 57-63.
- G. Voelcker, Anticancer Drugs 2019, 30, 435-440.
- C. Iyer, A. Kosters, G. Sethi, A. B. Kunnumakkara, B. B. Aggarwal, J. Versalovic, Cell Microbiol 2008, 10, 1442-1452.
- P. S. Schwartz, D. J. Waxman, Mol Pharmacol 2001, 60, 1268-1279.
- M. Hassan, B. S. Andersson, Pharmacogenomics 2013, 14, 75-87.
- I. El-Serafi, P. Afsharian, A. Moshfegh, M. Hassan, Y. Terelius, PLoS One 2015, 10, e0141979.




