Published 2018-03-26
Keywords
- sodium pump,
- active transport,
- discovery,
- physiological role,
- structure
How to Cite
Abstract
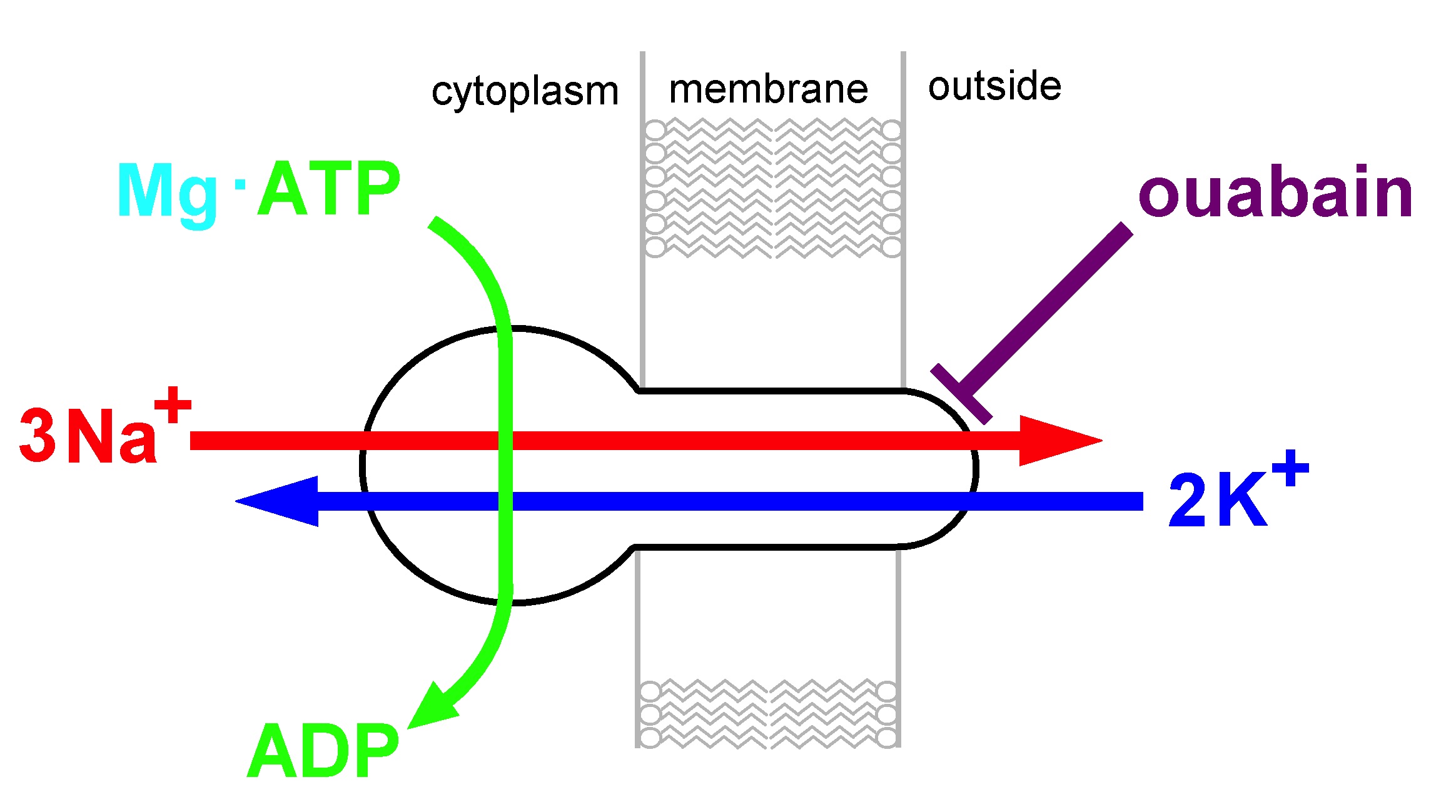
The oppositely oriented concentration gradients of Na+ and K+ ions across the cell membrane as found in animal cells led to the requirement of an active ion-transport mechanism that maintains this steady-state condition. As solution of this problem the Na,K-ATPase was identified, a member of the P-type ATPase family. Its stoichiometry has been defined as 3 Na+/2 K+/1 ATP, and a class of Na,K-ATPase-specific inhibitors, cardiac steroids, was established, which allow the identification of this ion pump. In an effort lasting for several decades structural details were uncovered down to almost atomic resolution. The quaternary structure of the functional unit, either ?? heterodimer or (??)n complexes with n ? 2, is still under discussion.





