Published 2021-03-01
Keywords
- Antisense,
- Oligonucleotide,
- Analogs,
- Therapeutic,
- RNA
How to Cite
Copyright (c) 2020 Jack S. Cohen

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
In the search for novel therapeutics, antisense oligonucleotide (ASO) analogs have been a major focus of research for over 40 years. They use the antisense strategy, namely they have a nucleic acid base sequence that is complementary to a portion of a specific mRNA that is produced in the cell, or to a viral RNA, in order to selectively inhibit gene expression. Oligonucleotides need to be chemically modified to stabilize them against hydrolysis by endogenous nucleases. Until now several phosphorothioate (PS) oligonucleotide analogs have been approved by the FDA for human use. This article seeks to provide a history of this subject to date.
References
- Jack S. Cohen, Informational drugs: a new concept in pharmacology, in New Leads and Targets in Drug Research, Bezon Fndtn. Symposium, Copenhagen, 1992, Vol. 33, pp. 17-32.
- Jack S. Cohen, Michael E. Hogan, The New Genetic Medicines, Scientific American 271, 77-82, 1994.
- Franklin H. Portugal, Jack S. Cohen, A Century of DNA: A history of the discovery of the structure and function of the genetic substance. MIT Press, 1977.
- Paul C. Zamecnik, Mary L. Stephenson, Inhibition of Rous sarcoma Virus Replication and Cell Transformation by a Specific Oligodeoxynucleotide, Proceedings of the National Academy of Sciences of the United States of America 75, 280-84, 1978.
- It should be mentioned that there was a prior publication that termed the antisense approach “complementarily addressed” from a group in the USSR, in which they used hexanucleotides attached to reactive groups to bind to and modify specific sites in tRNA. See section on covalently linked nucleic acids.
- I use the term "oligo" to mean any oligonucleotide and ASO to refer to an antisense oligonucleotide
- P. S. Eder, R. J. DeVine, J. M. Dagle, J. A. Walder, Substrate specificity and kinetics of degradation of antisense oligonucleotides by a 3' exonuclease in plasma Antisense Research and Development, 141-51, 1991.
- Reino Heikkila, Gisela Schwab, Eric Wickstrom, Shee Loong Loke, Dov H. Pluznik, Rosemary Watt, Leonard M. Neckers, A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1, Nature 328, 445-9, 1987.
- Louis J. Maher, Bruce J. Dolnick, Specific hybridization arrest of dihydrofolate reductase mRNA in vitro using anti-sense RNA or anti-sense oligonucleotides, Archives of Biochemistry and Biophysics 253, 214-20, 1987.
- I published a fictionalized account of my experiences in my book "Antisense" in 2014.
- Cheryl H. Agris, Kathleen R. Blake, Paul S. Miller, M. Parameswara Reddy, Paul O. P. Ts'o, Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates, Biochemistry 25, 6268-75, 1986.
- Paul S. Miller, Cheryl H. Agris, Laure Aurelian, Kathleen R. Blake, Shwu Bin Lin, Akira Murakami, M. Parameswara Reddy, Cynthia Smith, Paul O. P. Ts'o, Control of gene expression by oligonucleoside methylphosphonates, Jerusalem Symposia on Quantum Chemistry and Biochemistry 18, 207-9, 1985.
- Krishna Jayaraman, Kevin McParland, Paul Miller, Paul O. P. Ts'o, Selective inhibition of Escherichia coli protein synthesis and growth by nonionic oligonucleotides complementary to the 3? end of 16S rRNA, Proi. Natl. Acad. Sci. 78, 1537-41, 1981.
- I had done my PhD in Cambridge University entitled “The Oxidation of Phosphites” under Michael Blackburn and Lord Todd.
- My Lab Chief Charles "Snuffy" Myers and my Division Chief Samuel Broder
- I had met Gerry Zon thru my post-doc Bill Egan, and when I was on sabbatical at the Weizmann Inst., Rehovot, Israel, in 1976-7, I agreed for him to use my laboratory at NIH for their joint research. Later in 1986 we shared a post-doc Kazuo Shinozuka.
- Marvin H. Caruthers, The Chemical Synthesis of DNA/RNA: Our Gift to Science, J. Biol. Chem. 288, 1420-27, 2013.
- W. Stec, G. Zon, W. Egan, Automated solid-phase synthesis, separation, and stereochemistry of phosphorothioate analogs of oligodeoxyribonucleotides, J. Am. Chem. Soc. 106, 6077-79, 1984.
- F. Eckstein, H. Gindl, Polyribonucleotides containing a phosphorothioate backbone, Europ. J. biochem. 13, 558-64 1970.
- S. Spitzer, F. Eckstein, Inhibition of deoxyribonucleases by phosphorothioate groups in oligodeoxyribonucleotides, Nucl. Acids Res. 16, 11691-704, 1988.
- C. A. Stein, C. Subasinghe, K. Shinozuka, J. S. Cohen, Physicochemical Properties of Phosphorothioate Oligodeoxynucleotides, Nucl. Acids Res. 16, 3209-21, 1988.
- C. Helene, J-J. Toulme, Control of Gene Expression by Oligodeoxynucleotides Covalently Linked to Intercalating Agents and Nucleic Acid-Cleaving Reagents, in Oligodeoxynucleotides: Antisense Inhibitors of Gene Expression, ed. by J. S. Cohen, Macmillan, 1989.
- The automated synthesis uses phosphoramidites at the PIII level of oxidation and these give >99% yield of joined product and this is then oxidized to phosphate. Sulfurization uses a solution of sulfur instead.
- M H. Caruthers, S L. Beaucage, C. Becker, J W. Efcavitch, E F. Fisher, G. Galluppi, R. Goldman, P. deHaseth, M. Matteucci, L. McBride, Deoxyoligonucleotide synthesis via the phosphoramidite method, Gene Amplification and Analysis 3, 1-26, 1983.
- Serge L. Beaucage, Radhakrishnan P. Iyer, Advances in the synthesis of oligonucleotides by the phosphoramidite approach, Tetrahedron 48, 2223-311, 1992.
- C. Ott, F. Eckstein, Protection of oligonucleotide primers agaisnt degradation by DNA polymerase, Biochemistry 26, 8237-41, 1987.
- Makoto Matsukura, Kazuo Shinozuka, Gerald Zon, Hiroaki Mitsuya, Marvin Reitz, Jack S. Cohen, Samuel Broder, Phosphorothioate Analogs of Oligodeoxynucleotides: Inhibitors of Replication and Cytopathic Effects of Human Immunodeficiency Virus Proc. Natl. Acad. Sci. 84, 7706-10, 1987.
- M. Matsukura, G. Zon, K. Shinozuka, M. Roberts-Guroff, C. A. Stein, H. Mitsuya, F. Wong-Staal, J. S. Cohen, S. A. Broder, Regulation of Viral Expression of HIV in vitro by Antisense Phosphorothioate Oligodeoxynucleotide against rev, Proc. Natl. Acad. Sci. 86, 4244-48, 1989.
- Carol Marcus-Sekura, Amy M. Woerner, Kazuo Shinozuka, Gerald Zon, Jr Gerald V. Quinnan, Comparative inhibition of chloramphenicol acetyltransferase gene expression by antisense oligonucleotide analogues having alkyl phosphotriester, methylphosphonate and phosphorothioate linkages, Nucl. Acids Res. 15, 5749–63, 1987.
- Sudhir Agrawal, John Goodchild, Maria P. Civeira, Arthur H. Thornton, Prem S. Sarin, Paul C. Zamecnik, Oligodeoxynucleoside Phosphoramidates and Phosphorothioates as Inhibitors of Human Immunodeficiency Virus Proc. Natl. Acad. Sci. 85, 7079-83, 1988.
- D. Kinchington, S. Galpin, J. W. Jaroszewski, K. Ghosh, C. Subasinghe, J. S. Cohen, A comparison of gag, pol and rev antisense oligodeoxynucleotides as inhibitors of HIV-1, Antivir. Res. 17, 53-62, 1992.
- D. Archambault, C. A. Stein, J. S. Cohen, Phosphorothioate oligonucleotides inhibit the replication of lentiviruses and type D retroviruses but not that of type C retroviruses, : , , Arch. Virology. 139, 97-109, 1994.
- S. L. Loke, Zhang, X. H., Stein, C. A., Avigan, M., Cohen, J. S., and Neckers, L. M., Delivery of c-myc antisense phosphorothioate oligodeoxynucleotides to hematapoietic cells in culture by liposome fusion, Curr. Top. Microbiol. Immunol. 141, 282-9, 1988.
- J. C. Reed, C. A. Stein, C. A. Subasinghe, S. Haldar, C. M. Croce, S. Yum, J. S. Cohen, Antisense-mediated inhibition of BCL2 proto-oncogene expression and leukemic cell growth; comparisons of phosophorothioate oligonucleotides, Cancer Res. 50, 6565-70, 1990.
- Michael Riordan of Menlo Ventures called me after Makoto Matsukura made our first preserntiaton at a conference in San Franciso and asked to come and see me, and he flew out that night. I recall being telephoned by him later at a restaurant in Chinatown, Washington DC, in 1987 to tell me that he had founded what became Gilead Sciences, now a b$22 company.
- I was invited to join several start-up companies, but I became initial scientific adviser to Gilead Sciences and Pharmagenics.
- The patents were "Inhibitors for the expression of viruses and oncogenes" (NIH: 5,264,423, and 5,276,019, 1993)
- Conference on "Oligodeoxynucleotides as Antisense Inhibitors of Gene Expression: Therapeutic Implications," Organizing Committee: Jack S. Cohen, James Cradock, Ira Green, George Johnson, Cathy Laughlin, Mace Rothenberg, Nava Sarver, Sheraton Potomac Inn, Rockville MD, June 18-21, 1989
- This was only the second open meeting held at the formerly closed Soviet city of Akademgorodok in the era of glasnost.
- Fritz Eckstein, Phosphorothioates, essential components of therapeutic oligonucleotides, Nucleic Acid Therapeutics 24, 374-87, 2014.
- Jack S. Cohen, Oligodeoxynucleotides: Antisense Inhibitors of Gene Expression. Macmillan, 1989, Topics in Molecular and Structural Biology Vol. 12.
- M. Ghosh, K. Ghosh, J. S. Cohen, Oligodeoxynucleotide phosphorothioate- phosphodiester co-polymers: assessment for antisense applications, Anticancer Drug Design 8, 15-32, 1993.
- L. Zhou, M. Morocho, B. C. Chen, J. S. Cohen, Synthesis of phosphorothioate-methylphosphonate oligonucleotide co-polymers, Nucl. Acids Res. 22, 453-56, 1994.
- S. Agrawal, Z. Jaing, D. Shaw, Q. Cai, A. Roskey, L. Channavajjala, C. Saxinger, R. Zhang, Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: In vitro and in vivo studies, Proc. Natl. Acad. Sci. 94, 2620-25, 1997.
- Joachim W. Engels, Fritz Eckstein, Antisense Oligonucleotides as Potential Drugs, Reviews in Cell Biology and Molecular Medicine, 2006.
- James W. Hawkins, A Brief History of Genetic Therapy, Gene Therapy , Antisense Technology and Genomics, in Cinical Trials of Genetic Therapy with Antisense DNA and DNA Vectors, ed. by E. Wickstrom, Marcel Dekker, 1998, pp. 1-38.
- D. M. Tidd, P. Hawley, H. M. Warenius, I. Gibson, Evaluation of N-ras oncogene anti-sense, sense and nonsense sequence methylphosphonate oligonucleotide analogs Anti-Cancer Drug Design 3, 117-27, 1988.
- F. Morvan, B. Rayner, J-L. Imbach, S. Thenet, J.R. Bertrand, J. Paoletti, C. Malvy, C. Paoletti, Synthesis of unnatural a-anomeric oligodeoxyribonucleotides containing the four usual bases and study of their suibstrate activities for nucleases, Nucl. Acids Res. 15, 3421-37, 1987.
- B. Rayner, M. Matsukura, F. Morvan, J.S. Cohen, J.-L Imbach, Activite anti-VIH in vitro d'oligodesoxynucleotides phosphorothioates d'anomerie alpha, C.R. Acad. Sci. Paris 310, 61-64, 1990.
- K. Mori, C. Boizeau, C. Cazenave, M. Matsukura, Subasinghe, C, J. S. Cohen, J. J. Toulme, C. A. Stein, Phosphoroselenoate oligodeoxynucleotides: synthesis, physico-chemical characterization, anti-sense inhibitory properties and anti-HIV activity, Nucl. Acids. Res 17, 8207-19, 1989.
- P S. Miller, J C. Barrett, P O. P. Ts'o, Synthesis of oligodeoxyribonucleotide ethyl phosphotriesters and their specific complex formation with transfer ribonucleic acid, Biochemistry 13, 4887-96, 1974.
- J. W. Jaroszewski, V. Clausen, J. S Cohen, O. Dahl, NMR investigations of duplex stability of phosphorothioate and phosphorodithioate DNA analogues modified in both strands, Nucl. Acids Res. 24, 829-34, 1996.
- Suzanne Peyrottes, Jean-Jacques Vasseur, Jean-Louis Imbach, Bernard Rayner, Oligodeoxynucleoside phosphoramidates (P-NH2): synthesis and thermal stability of duplexes with DNA and RNA targets, Nucl. Acids Res. 24, 1841-48 1996.
- J. W. Jaroszewski, J. S. Cohen, Cellular Uptake of Antisense Oligodeoxynucletides., in Advanced Drug Delivery Reviews, ed. by R. L. Juliano, 1991, pp. 235-50.
- V. Meidan, J. S. Cohen, N Amariglio, D. Hirsch-Lerner, Y. and Barenholz, Interaction of oligonucleotides with cationic lipids: the relationship between electrostatics, hydration and state of aggregation, Biochim. Biophys. Acta. 1464, 251-61, 2000.
- V. Meidan, J. Glazer, Amariglio, N., J. Cohen, Y. Barenholz, Oligonucleotide lipoplexes: the influence of oligonucleotide composition on complexation, Biochim Biophys Acta. 1568, 177-82, 2001.
- V. Meidan, J. Glazer, S. Salomon, Y. Sidi, Y. Barenholz, J. S. Cohen, G. Lilling, Antisense against Bcl-2 in breast cancer cells: A systematic comparison of different lipoplexes, J. Liposome Res. 16, 27-43, 2006.
- Michael J. Gait, Peptide-mediated cellular delivery of antisense oligonucleotides and their analogues, Cellular and Molecular Life Sciences 60, 844-53, 2003.
- Nathaniel L. Rosi, David A. Giljohann, C. Shad Thaxton, Abigail K. R. Lytton-Jean, Min Su Han, Chad A. Mirkin, Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation, Science, 1027-30, 2006.
- S. Akhtar, (ed.), Delivery strategies for antisense oligonucleotide therapeutics. CRC Press, 1995/2017.
- Stanley T. Crooke, Shiyu Wang, Timothy A. Vickers, Wen Shen, Xue-Hai Liang, Cellular uptake and trafficking of antisense oligonucleotides, Nature biotechnology 35, 230-37, 2017.
- Radhakrishnan P. Lyer, Bogdan Uznanski, Jila Boal, Christy Storm, William Egan, Makoto Matsukura, Samue Broder, Gerald Zon, Andrzej Wilk, Abasic oligodeoxyribonucleoside phosphorothioates: synthesis and evaluation as anti-HIV-1 agents Nucl, Acids Res. 18, 2855-59, 1990.
- W. Gao, J. W. Jaroszewski, J. S. Cohen, Y.-C. Cheng, Mechanisms of inhibition of herpes simplex virus type 2 growth by 28-mer phosphorothioate oligodeoxycytidine, J. Biol. Chem. 33, 20172-178 1990.
- K. B. Spurgers, C. M. Sharkey, K. L. Warfield, S. Bavari, Oligonucleotide antiviral therapeutics: Antisense and RNA interference for highly pathogenic RNA viruses, Antiviral Research 78, 26-36, 2008,.
- Chiara Brignole, Fabio Pastorino, Danilo Marimpietri, Gabriella Pagnan, Angela Pistorio, Theresa M. Allen, Vito Pistoia, Mirco Ponzoni, Immune Cell-Mediated Antitumor Activities of GD2-Targeted Liposomal c-myb Antisense Oligonucleotides Containing CpG Motifs, Journal of the National Cancer Institute 96, 1171-80 2004.
- Volker Wacheck, Clemens Krepler, Sabine Strommer, Elisabeth Heere-Ress, Robert Klem, Hubert Pehamberger, Hans-Georg Eichler, Burkhard Jansen, Antitumor effect of G3139 Bcl-2 antisense oligonucleotide is independent of its immune stimulation by CpG motifs in SCID mice, Antisense & Nucleic Acid Drug Development 12, 359-67 2002.
- Husam S. Younis, Tim Vickers, Arthur A. Levin, Scott P. Henry, CpG and non-CpG oligodeoxynucleotides induce differential proinflammatory gene expression profiles in liver and peripheral blood leukocytes in mice, Journal of Immunotoxicology 3, 57-68 2006.
- John M. Dagle, Joseph A. Walder, Daniel L. Weeks, Targeted degradation of mRNA in Xenopus oocytes and embryos directed by modified oligonucleotides: studies of An2 and cyclin in embryogenesis, Nucl. Acids Res. 18, 4751–57, 1990.
- R Y Walder, J A Walder, Role of RNase H in hybrid-arrested translation by antisense oligonucleotides, Proc. Natl. Acad. Sci. Jul; (14): 85, 5011-15, 1988.
- S. Agrawal, S.H. Mayrand, P.C. Zamecnik, T. Pederson, Site-specific excision from RNA by RNase H and mixed-phosphate backbone oligodeoxynucletides, Proc. Natl. Acad. Sci. 87, 1401-5, 1990.
- Gareth J. Veal, Sudhir Agrawal, Randal A. Byrn, Sequence-specific RNase H cleavage of gag mRNA from HIV-1 infected cells by an antisense oligonucleotide in vitro, Nucl. Acids Res. 26, 5670-75, 1998.
- Wen Yi Gao, Fu Sheng Han, Christy Storm, William Egan, Yung Chi Cheng, Phosphorothioate oligonucleotides are inhibitors of human DNA polymerases and RNase H: Implications for antisense technology, Molecular Pharmacology 41, 223-29, 1992.
- J. Summerton, Morpholino antisense oligomers: the case for an RNase H-independent structural type, Biochimica et Biophysica Acta, Gene Structure and Expression 1489, 141-58, 1999.
- Karen L. Fearon, Bernard L. Hirschbein, Choi-Ying Chiu, Maria R. Quijano, Gerald Zon, Phosphorothioate oligodeoxynucleotides: large-scale synthesis and analysis, impurity characterization, and the effects of phosphorus stereochemistry, Ciba Foundation Symposium 209, 19-37, 1997.
- Ilyas M. Khan, Judy M. Coulson, A novel method to stabilize antisense oligonucleotides against exonuclease degradation, Nucl. Acids Res. 21, 2957-8, 1993.
- Grzegorz Rebowski, Marzena Wojcik, Malgorzata Boczkowska, Edyta Gendaszewska, Miroslaw Soszynski, Grzegorz Bartosz, Wojciech Niewiarowski, Antisense hairpin loop oligonucleotides as inhibitors of expression of multidrug resistance-associated protein 1: their stability in fetal calf serum and human plasma, Acta Biochimica Polonica 48, 1061-76, 2001.
- A. Fire, S. Xu, MK. Montgomery, SA. Kostas, Se. Driver, CC. Mello, Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans, Nature 391, 806-11, 1998.
- P. D. Zamore, T. Tuschi, P.A. Sharp, D Bartel, P, RNAi: Double stranded RNA directs the ATP-dependent cleavage of mRNA at 21-23 nucleotide intervals, Cell 101, 25-53, 2000.
- Y. Dorsett, T. Tuschi, siRNAs: Applications in functional genomics and potential as therapeutics, Natrure Reviews 3, 318-29, 2004.
- Mark A. Behlke, Chemical Modification of siRNAs for In Vivo Use, Oligonucleotides 18, 305-20, 2008.
- R. Titze-de-Almeida, C. David, S.S. Titze-de-Almeida, The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market, Pharm. Res. 34, 1339–63, 2017.
- GF. Deleavey, JK. Watts, T. Alain, Synergistic effects between analogs of DNA and RNA inprove the potency of si-RNA mediated gene silencing, Nucl. Acids Res. 38, 4547-57, 2010.
- Guihua Sun, John J. Rossi, Problems associated with reporter assays in RNAi studies, RNA Biology 6, 406-11, 2009.
- Frank Vandendriessche, Koen Augustyns, Van Aerschot, Roger Busson, Jos Hoogmartens, Piet Herdewijn, Acyclic oligonucleotides: possibilities and limitations, Tetrahedron 49, 7223-38, 1993.
- Michael Egholm, Ole Buchardt, Peter E. Nielsen, Rolf H. Berg, Peptide nucleic acids (PNA). Oligonucleotide analogs with an achiral peptide backbone, J. Am. Chem. Soc. 224, 1895-7, 1992.
- Jesper Wengel, Alexei Koshkin, Sanjay K Singh, Poul Nielsen, Michael Meldgaard, Vivek K. Rajwanshi, Ravindra Kumar, Jan Skouv, Christina B Nielsen, Jens Peter Jacobsen, LNA (Locked Nucleic Acid), Nucleosides & Nucleotides 18, 1365-70, 1999.
- J Summerton, D. Weller, Morpholino antisense oligomers: design, preparation, and properties, Antisense & Nucleic Acid Drug Development 7, 187-95, 1997.
- E; Wagner, B Oberhauser, A Holzner, H Brunar, G Issakides, G Schaffner, M Cotten, M Knollmüller, C R. Noe, A simple procedure for the preparation of protected 2'-O-methyl or 2'-O-ethyl ribonucleoside-3'-O-phosphoramidites, Nucl. Acids Res. 19, 5965-71, 1991.
- David B. Olsen, Fritz Benseler, Helle Aurup, Wolfgang A. Pieken, Fritz Eckstein, Study of a hammerhead ribozyme containing 2'-modified adenosine residues, Biochemistry 30, 9735-41, 1991.
- Yves Merle, Eric Bonneil, Liliane Merle, Janos Sagi, Attila Szemzo, Acyclic oligonucleotide analogs, International Journal of Biological Macromolecules 17, 239-46, 1995.
- K. Augustyns, J. Rozenski, A. Van Aerschot, G. Janssen, P. Herdewijn, Synthesis of 2,4-dideoxy-.beta.-D-erythro-hexopyranosyl nucleosides, J. Org. Chem. 58, 2977-82, 1991.
- E. Meggers, L. Zhang, Synthesis and properties of the simplified nucleic acid glycol nucleic acid, Accts. Chem. Res. 43, 1092-102, 2010.
- AT. Johnson, MK. Schlegel, E. Meggers, LO. Essen, O. Wiest, On the structure and dynamics of duplex GNA, J. Org. Chem., 7964-74, 2011.
- N. I. Grineva, G. G Karpova, Complementarily addressed modification of rRNA with p-(chloroethylmethylamino)benzylidene hexanucleotides, FEBS Letters 32, 351-5, 1973.
- U. Asseline, M. Delarue, G. Lancelot, J-J. Toulme, N.T. Thuong, T. Montenay-Garestier, C. Helene, Nucleic acid-binding moleculares with high affinity and base sequence specificity: Intercalating agents covalently linked to oligodeoxynucleotides, Proc. Natl. Acad. Sci. 81, 3297-301, 1984.
- C. A. Stein, K. Mori, S. L. Loke, C. Subasinghe, K. Shinozuka, J. S. Cohen, L. M. Neckers, Phosphorothioate and Normal Oligodeoxynucleotides with 5'-linked Acridine: Characterization and Preliminary Kinetics of Cellular Uptake, Gene 72, 333-41, 1988.
- A.S. Boutorin, V.V. Vlassov, S.A. Kazakov, I.V. Kutiavin, M.A. Podyminogin, Complementary addressed reagents carrying EDTA-Fe(II) groups for drected cleavage of single-stranded nucleic acids, FEBS Letters 172, 43-46, 1984.
- B.C.F. Chu, L.E. Orgel, Nonenzymatic sequence-specific cleavage of single-stranded DNA, Proc. Natl. Acad. Sci. 82, 963-67, 1985.
- G.B Dreyer, P.B. Dervan, Sequence-specific cleavage of single stranded DNA: Oligodeoxynucleotides-EDTA-Fe(II), Proc. Natl. Acad. Sci. 82, 968-72, 1985.
- T. Le Doan, L. Perroualt, M. Chassignol, N.T Thuong, C. Hele3ne, Sequence-targeted chemical modifications of nucleic acids by complementary oligonucleotides covalently liked to porphyrin, Nucl. Acids Res. 15, 8643-59, 1987.
- D.G. Knorre, V.V. Vlassov, V.F. Zarytova, Oligodeoxynucleotides linked to reactive groups, in Oligodeoxynucleotides, ed. by J. S. Cohen, Macmillan, 1989, pp. 173-96.
- N. N. Polushin, B.-C. Chen, L. W. Anderson, J. S. Cohen, Synthesis and characterization of imidazoyl-linked synthons and 3'-conjugated thymidine derivatives, J. Org. Chem. 58, 4606-13,, 1993.
- G. C. K. Roberts, E. A. Dennis, D. H. Meadows, J. S. Cohen, O. Jardetzky, The Mechanism of Action of Ribonuclease, Proc. Natl. Acad. Sci. USA 62, 1151-8, 1969.
- M. Beban, P. S. Miller, Preparation of an imidazole-conjugated oligonucleotide, Bioconjug. Chem 11, 599-603,, 2000.
- N. G. Beloglazova, V. N. Sil'nikov, M. A. Zenkova, V. V. Vlassov, Cleavage of yeast tRNAPhe with complementary oligonucleotide conjugated to a small ribonuclease mimic, FEBS Letters 481, 277-80, 2000.
- K. Ushijima, H. Gouzu, K. Hosono, M. Shirakawa, K. Kagosima, K. Takai, H. Takaku, Site-specific cleavage of tRNA by imidazole and/or primary amine groups bound at the 5'-end of oligodeoxyribonucleotides, Biochim Biophys Acta. 1379, 217-23, 1998.
- N. G. Beloglazova, M. M. Fabani, M. A. Zenkova, E. V. Bichenkova, N. N. Polushin, V. V. Sil’nikov, K. T. Douglas, V. V. Vlassov, Sequence-specific artificial ribonucleases. I. Bis-imidazole-containing oligonucleotide conjugates prepared using precursor-based strategy, Nucleic Acids Research 32, 3887–97, 2004.
- N. G. Beloglazova, M. M. Fabani, N. N. Polushin, V. V. Sil’nikov, V. V. Vlassov, E. V. Bichenkova, M. A. Zenkova, Site-Selective Artificial Ribonucleases: Oligonucleotide Conjugates Containing Multiple Imidazole Residues in the Catalytic Domain, Journal of Nucleic Acids, Article ID 748632, 2011.
- M. Mondhe, A. Chessher, S. Goh, L. Good, J.E.M. Stach, Species-Selective Killing of Bacteria by Antimicrobial Peptide-PNAs., Plos One 9, e89082, 2014.
- N. Patenge, R. Pappesch, F. Krawack, C. Walda, M. Abu Mraheil, A. Jacob, T. Hain, B. Kreikemeyer, Inhibition of Growth and Gene Expression by PNA-peptide Conjugates in Streptococcus pyogenes, Mol. Therapy—Nucl. Acids 2, e132, 2013.
- Natalia Nekhotiaeva, Satish Kumar Awasthi, Peter E. Nielsen, Liam Good, Inhibition of Staphylococcus aureus gene expression and growth using antisense peptide nucleic acids, Molecular Therapy 10, 652-59 2004.
- Jens Kurreck, Eliza Wyszko, Clemens Gillen, Volker A. Erdmann, Design of antisense oligonucleotides stabilized by locked nucleic acids, Nucl. Acids Res. 30, 1911-18, 2002.
- Claes Wahlestedt, Peter Salmi, Liam Good, Johanna Kela, Thomas Johnsson, Tomas Hokfelt, Christian Broberger, Frank Porreca, Josephine Lai, Kunkun Ren, Potent and nontoxic antisense oligonucleotides containing locked nucleic acids, Proceedings of the National Academy of Sciences of the United States of America 97, 5633-38, 2000.
- James Summerton, David Stein, Sung Ben Huang, Paula Matthews, Swight Weller, Michael Partridge, Morpholino and phosphorothioate antisense oligomers compared in cell-free and in-cell systems, Antisense & Nucleic Acid Drug Development 7, 63-70 1997.
- Shantanu Karkare, Deepak Bhatnagar, Promising nucleic acid analogs and mimics: characteristic features and applications of PNA, LNA, and morpholino, Applied Microbiology and Biotechnology 71, 575-86 2006.
- B. W. Neuman, D. A. Stein, A. D. Kroeker, M.J. Churchill, A. M. Kim, P. Kuhn, P. Dawson, H. M. Moulton, R. K. Bestwick, P. L. Iversen, Inhibition, escape, and attenuated growth of severe acute respiratory syndrome coronavirus treated with antisense morpholino oligomers, Journal of virology 79, 9665-76 2005.
- A. M. Iribarren, B. S. Sproat, P. Neuner, I. Sulston, U. Ryder, A. I. Lamond, 2'-O-alkyl oligoribonucleotides as antisense probes, Proc. Natl. Acad. Sci. 87, 7747-51, 1990.
- Akila Mayeda, Yoji Hayase, Hideo Inoue, Eiko Ohtsuka, Yasumi Ohshima, Surveying Cis-Acting Sequences of Pre-mRNA by Adding Antisense 2?-O-Methyl Oligoribonucleotides to a Splicing Reaction, The Journal of Biochemistry 108, 399–405, 1990,.
- A I. Lamond, B S. Sproat, Antisense oligonucleotides made of 2'-O-alkylRNA: their properties and applications in RNA biochemistry, FEBS letters 325, 123-7, 1993.
- Brett P. Monia, Elena A.; Gonzalez Lesnik, Carolyn, Walt F. Lima, Danny McGee, Charles J. Guinosso, Andrew M. Kawasaki, P. Dan Cook, Susan M Freier, Evaluation of 2'-modified oligonucleotides containing 2'-deoxy gaps as antisense inhibitors of gene expression, J. Biol. Chem. 268, 14514-22, 1993.
- Valeri Metelev, Julianna Lisziewicz, Sudhir Agrawal, Study of antisense oligonucleotide phosphorothioates containing segments of oligodeoxynucleotides and 2'-O-methyloligoribonucleotides, Bioorganic & Medicinal Chemistry Letters 4, 2929-34, 1994.
- Byong Hoon Yoo, Elena Bochkareva, Alexey Bochkarev, Tung-Chung Mou, Donald M Gray, 2'-O-methyl-modified phosphorothioate antisense oligonucleotides have reduced non-specific effects in vitro, Nucl. Acids Res. 32, 2008-16 2004.
- A. Artsma-Rus, W.E. Kaman, M. Bremer-Bout, A. A. Janson, J. T. den Dunnan, G .J. van Ommen, J. C. van Deutekom, Comparative analysis of antisense oligonucletide analogs for targeted DMD exon 46 skipping in muscle cells, Gene Therapy 11, 1391-8, 2004.
- Richard V. Giles, David M. Tidd, Enhanced RNase H activity with methylphosphonodiester/phosphodiester chimeric antisense oligodeoxynucleotides, Anti-Cancer Drug Design 7, 37-48, 1992.
- A M Kawasaki, M D Casper, S M Freier, E A Lesnik, M C Zounes, L L Cummins, C Gonzalez, P D Cook, Uniformly modified 2'-deoxy-2'-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets, Journal of Medicinal Chemistry 36, 831-41, 1993.
- A. Robert MacLeod, Stanley T. Crooke, RNA Therapeutics in Oncology: Advances, Challenges, and Future Directions, The Journal of Clinical Pharmacology 57, S43–S59, 2017.
- K.-H. Altmann, D. Fabbrot, N. M. Dean, T. Geigert, 6. P. Mania, M. Mullert, P. Nickling, Second-generation antisense oligonucleotides: structure-activity relationships and the design of improved signal-transduction inhibitors, Biochemical Society Transactions 24, 630-37, 1996.
- C. Frank Bennett, Stanley T. Crooke, Muthiah Manoharan, et al., Alteration of cellular behavior by antisense modulation of mRNA processing, United States Patent, US20020049173 2002.
- A. R. Morgan, R. D. Wells, Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences, Journal of Molecular Biology 37, 63-80, 1968.
- L. J. Maher, B. Wold, P. B. Dervan, Oligonucleotide-directed DNA triple-helix formation: an approach to artificial repressors?, Antisense research and development 1, 277-81, 1991.
- H. E. Moser, P. B. Dervan, Sequence-specific cleavage of double helical DNA by triple helix formation, Science 238, 645-50 1987.
- C. Helene, The Anti-Gene Strategy: control of gene expression by triplex-forming oligonucleotides, Anticancer Drug Design 6, 569-84, 1991.
- Karen M. Vasquez, Theodore G. Wensel, Michael E. Hogan, John H. Wilson, High-Affinity Triple Helix Formation by Synthetic Oligonucleotides at a Site within a Selectable Mammalian Gene, Biochemistry 34, 7243-51 1995.
- John Abelson, Directed evolution of nucleic acids by independent replication and selection, Science 249, 488-9, 1990.
- Andrew D. Ellington, Jack W. Szostak, In vitro selection of RNA molecules that bind specific ligands, Nature 346, 818-22 1990.
- Craig Tuerk, Larry Gold, Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase, Science 249, 505-10, 1990.
- Petra Burgstaller, Michael Famulok, Isolation of RNA aptamers for biological cofactors by in vitro selection, Angew. Chem., Int. Ed. Engl. 33, 1084-7, 1994.
- Rick Conrad, Lisa M. Keranen, Andrew D. Ellington, Alexandra C. Newton, Isoenzyme-specific inhibition of protein kinase C by RNA aptamers, J. Biol. Chem. 269, 32051-4, 1994.
- D. E. Huizenga, J. W. Szostak, A DNA aptamer that binds adenosine and ATP, Biochemistry 34, 656-65, 1995.
- Roman F. Macaya, Peter Schultze, Flint W. Smith, James A. Roe, Juli Feigon, Thrombin-binding DNA aptamer forms a unimolecular quadruplex structure in solution, Proc. Natl. Acad. Sci. 90, 3745-9, 1993.
- M. L. Andreola, C. Calmels, J. Michel, J. J. Toulmé, S. Litvak, Towards the selection of phosphorothioate aptamers optimizing in vitro selection steps with phosphorothioate nucleotides, Europ. J. Biochem. 267, 5032-40, 2000.
- C. Boiziau, J. J. Toulmé, A method to select chemically modified aptamers directly, Antisense & Nucleic Acid Drug Development 11, 379-85, 2001.
- Joon-Hwa Lee, Marella D. Canny, Andrea De Erkenez, Dominik Krilleke, Yin-Shan Ng, David T.. Shima, Arthur Pardi, Fiona Jucker, A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165, Proc. Natl. Acad. Sci. 102, 18902-07 2005.
- Brenda L. Bass, Thomas R. Cech, Specific interaction between the self-splicing RNA of Tetrahymena and its guanosine substrate: implications for biological catalysis by RNA, Nature 308, 820-6, 1984.
- Cecilia Guerrier-Takada, Katheleen Gardiner, Terry Marsh, Norman Pace, Sidney Altman, The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme, Cell 35, 849-57, 1983.
- K. Kruger, P.J. Grabowski, A.J. Zaug, J. Sands, D.E. Gottschling, T.R. Cech, Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena, Cell 31 147–57, 1982.
- M.J. Fedor, J.R. Williamson, The catalytic diversity of RNAs, Nature Reviews Molecular Cell Biology 6 399–412, 2005.
- Sidney Altman, RNA enzyme-directed gene therapy, Proc. Natl. Acad. Sci. 90, 10898-900, 1993.
- David J. Earnshaw, Michael J. Gait, Progress toward the structure and therapeutic use of the hairpin ribozyme, Antisense & Nucleic Acid Drug Development 7, 403-11, 1997.
- A. Thybusch-Bernhardt, A. Aigner, S. Beckmann, F. Czubayko, H. Juhl, Ribozyme targeting of HER-2 inhibits pancreatic cancer cell growth in vivo, European Journal of Cancer 37, 1688-94, 2001.
- J. Jaroszewski, J.L. Syi, M. Ghosh, K. Ghosh, J. S. Cohen, Targeting of antisense DNA: comparison of activity of anti-rabbit b-globin phosphorothioate oligodeoxynucleotides with computer predictions of mRNA folding, Antisense Res. Devel. 3, 339-48, 1993.
- Philippe Verspieren, Nadine Loreau, Nguyen Thanh Thuong, David Shire, Jean Jacques Toulme, Effect of RNA secondary structure and modified bases on the inhibition of trypanosomatid protein synthesis in cell free extracts by antisense oligodeoxynucleotides, Nucleic Acids Research 18, 4711-17, 1990.
- Vladislav A. Petyuk, Marina A. Zenkova, Richard Giege, Valentin V Vlassov, Hybridization of antisense oligonucleotides with the 3' part of tRNAPhe, FEBS Letters 444, 217-21, 1999.
- Ye Ding, Charles E. Lawrence, A statistical sampling algorithm for RNA secondary structure prediction, Nucl. Acids Res. 31, 7280-301 2003.
- J. W. Jaroszewski, J.-L. Syi, J. Maizel, J. S. Cohen, Towards rational design of antisense DNA: molecular modeling of phosphorothioate DNA analogues,, Anticancer Drug Design 7, 253-62, 1992.
- R. Soliva, E. Sherer, F.J. Luque, C.A. Laughton, M. Orozco, Molecular Dynamics Simulations of PNA·DNA and PNA·RNA Duplexes in Aqueous Solution J. Am. Chem. Soc. 122,, 5997-6008, 2000.
- R. Galindo-Murillo, D.R. Roe, T.E.I. Cheatham, Convergence and reproducibility in molecular dynamics simulations of the DNA duplex d(GCACGAACGAACGAACGC), Biochim. et Biophys. Acta (General Subjects) 1850,, 1041-58, 2015.
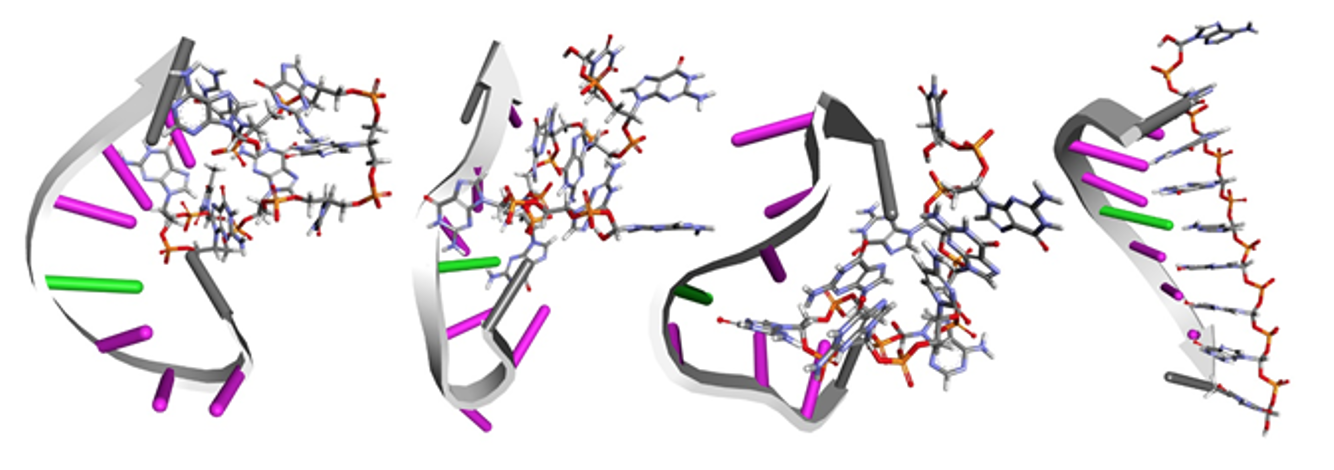
- Rodrigo Galindo-Murillo, Barak Akabayov, Jack S. Cohen, Molecular Dynamics Simulations of Duplexation of Acyclic Analogs of Nucleic Acids for Antisense Inhibition submitted for publication, 2020.
- Hui Wang, Jie Hang, Zhenqi Shi, Mao Li, Dong Yu, Ekambar R Kandimalla, Sudhir Agrawal, Ruiwen Zhang, Antisense oligonucleotide targeted to RIalpha subunit of cAMP-dependent protein kinase (GEM231) enhances therapeutic effectiveness of cancer chemotherapeutic agent irinotecan in nude mice bearing human cancer xenografts: in vivo synergistic activity, pharmacokinetics and host toxicity, International Journal of Oncology 21, 73-80, 2002.
- Ruiwen Zhang, Hui Wang, Sudhir Agrawal, Novel antisense anti-MDM2 mixed-backbone oligonucleotides: proof of principle, in vitro and in vivo activities, and mechanisms Current Cancer Drug Targets 5, 43-49, 2005.
- Xin-xia Zhang, Chang-cong Cui, Xiang-guang Xu, Xue-song Hu, Wei-hua Fang, Bi-juan Kuang, In vivo distribution of c-myc antisense oligodeoxynucleotides local delivered by gelatin-coated platinum-iridium stents in rabbits and its effect on apoptosis, Chinese Medical Journal (English Edition) 117, 258-63, 2004.
- Jacqueline Ruger, Silvia Ioannou, Daniela Castanotto, Cy A. Stein, Oligonucleotides to the (Gene) Rescue: FDA Approvals 2017–2019, Trends in Pharmacological Sciences 41, 27-41, 2020.
- Annemieke Aartsma-Rus, FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides Nucleic Acid Therapeutics 27, 67-69, 2017.
- Matthias Boentert, Stephan Wenninger, Valeria A. Sansone, Respiratory involvement in neuromuscular disorders, Current Opinion in Neurology 30, 529-37, 2017.
- W.P. Kloosterman, A.K. Lagendijk, R.F. Ketting, J.D. Moulton, R.H. Plasterk, Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development, PLOS Biology 5, e203, 2007.
- G. Meister, M. Landthaler, Y. Dorsett, T. Tuschl, Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing, RNA Biology 10 544–50, 2004.




