Chromosome, ploidy analysis, and flow cytometric genome size of caper (Capparis spinosa) medicinal plant
DOI:
https://doi.org/10.36253/caryologia-3633Keywords:
caper, Capparis spinosa, chromosome, karyology, 2Cx DNA, genome size, flow cytometryAbstract
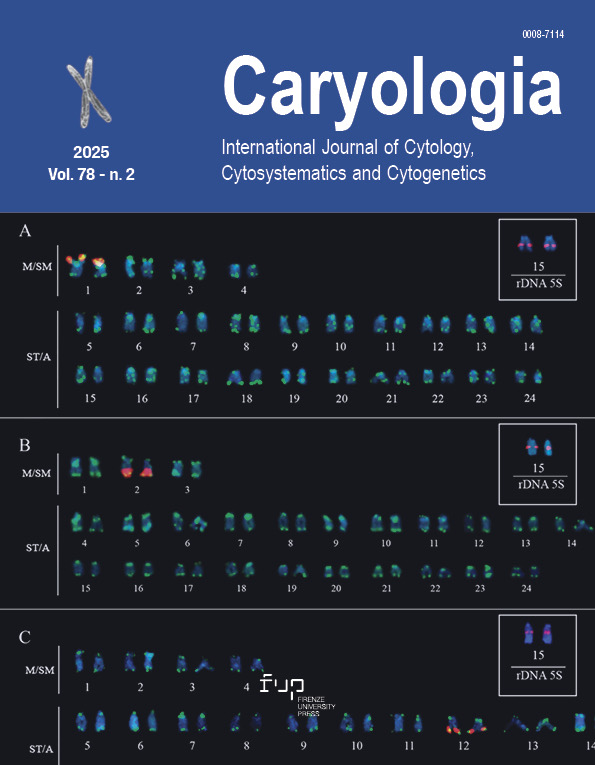
Caper (Capparis spinosa) is a shrubby, deciduous perennial medicinal plant belonging to the Capparaceae family. Its use is in folk medicine, pharmacy, food, and spices. Chromosome, ploidy analysis, and flow cytometric genome size of 10 populations collected from different parts of Iran were analyzed. The results showed that all populations were diploid, with nine populations having 2n = 2x = 30 (P1-P8, P10) and one population having 2n = 2x = 34 (P9) chromosomes. The average chromosome length (CL) for these two chromosome groups was 1.05 and 0.97 μm, respectively. The mean monoploid genome sizes for the populations with 30 and 34 chromosomes were 0.646 and 0.633 pg, respectively. As a whole, the mean genome size of all populations was 0.643 pg. The chromosome number as well as the genome size are being reported for the first time. Cluster analysis and principal component analysis revealed a categorization of the caper population into four distinct groups. The first group comprised three populations (P1, P3, and P4), while the second group included only P2 population, the third group was represented by two populations (P5 and P7), and the fourth group encompassed four populations (P6, P8, P9, and P10). Future research on the genetic traits and breeding methodologies of this species can build upon the foundational findings of this study.
Downloads
References
Abbasi-Karin Sh, Karimzadeh G, and Mohammadi-Bazargani M. 2022. Interspecific chromosomal and genome size variations in in vitro propagated willow herb (Epilobium spp.) medicinal plant. Cytologia 87(2): 129-135. https://doi.org/10.1508/cytologia.87.129.
Abedi R, Babaei A, and Karimzadeh G. 2015. Karyological and flow cytometric studies of Tulipa (Liliaceae) species from Iran. Plant Syst. Evol. 301: 1473-1484. https://doi.org/10.1007/s00606-014-1164-z.
Agah F. Esmaeili M. A. Farzam M. and Abbasi R. 2020. Effect of dormancy breaking treatments and seed bed medium on seed germination and morphology of Capparis spinosa L. Seedlings. Iran. J. Seed Sci. Technol. 9(3): 45-57. https://doi.org/10.22034/ijsst.2019.125542.1262.
Aguilar-Rito M. G. Arzate-Fernández A. M. García-Núñez, H. G. and Norman-Mondragón T. H. 2023. Establishment of an efficient protocol for in vitro disinfection of seeds of seven Agave spp. species. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 42. https://doi.org/10.18781/r.mex.fit.2310-1.
Ahmadi-Roshan M, Karimzadeh G, Babaei A, and Jafari H. 2016. Karyological studies of Fritillaria (liliaceae) species from Iran. Cytologia (Tokyo). 81: 133-141. https://doi.org/10.1508/cytologia.81.133.
Akbarzadeh M,Van Laere K, Leus L, De Riek J, Van Huylenbroeck J, Werbrouck SP, and Dhooghe E. 2021. Can knowledge of genetic distances, genome sizes and chromosome numbers support breeding programs in hardy geraniums? Genes (Basel). 12. https://doi.org/10.3390/genes12050730.
Ali MES, and Amar MH. 2020. A systematic revision of Capparaceae and Cleomaceae in Egypt: an evaluation of the generic delimitations of Capparis and Cleome using ecological and genetic diversity. J. Genet. Eng. Biotechnol. 18: 58. https://doi.org/10.1186/s43141-020-00069-z.
Al-Turki TA, Filfilan SA, and Mehmood SF. 2000. A cytological study of flowering plants from Saudi Arabia. Willdenowia 30: 339-358. https://doi.org/10.3372/wi.30.30211.
Anjum N, Dash CK, and Sultana SS. 2023. Karyological diversity among six medicinally important species of Acanthaceae with three new chromosome counts. Nucl., 1-9. https://doi.org/10.1007/s13237-023-00443-5.
Annaz H, Sane, Y, Bitchagno GTM, Ben Bakrim W, Drissi B, Mahdi I, El Bouhssini M, and Sobeh M. 2022. Caper (Capparis spinosa L.): An updated review on its phytochemistry, nutritional value, traditional uses, and therapeutic potential. Front. Pharmacol. 13: 1-22. https://doi.org/10.3389/fphar.2022.878749.
Ara KM, Karami M, and Raofie F. 2014. Application of response surface methodology for the optimization of supercritical carbon dioxide extraction and ultrasound-assisted extraction of Capparis spinosa. Elsevier. https://doi.org/10.1016/j.supflu.2013.10.016.
Argentieri M, Macchia F, Papadia P, Fanizzi F P, and Avato P 2012. Bioactive compounds from Capparis spinosa subsp. rupestris. Ind. Crops Prod. 36(1): 65-69. doi.org/10.1016/j.indcrop.2011.08.007.
Aydın ZU, Eroğlu HE, Şenova MK, Martin E, Tuna M, and Dönmez AA. 2024. Chromosome characterization and genome size in Nigella and Garidella species (Ranunculaceae) with their taxonomic implications. Cytologia 89: 117-125. https://doi.org/10.1508/cytologia.89.117.
Bakr RO, and El Bishbishy MH. 2016. Profile of bioactive compounds of Capparis spinosa var. aegyptiaca growing in Egypt. Rev. Bras. Farmacogn. 26: 514-520. https://doi.org/10.1016/J.BJP.2016.04.001.
Barbera G, and Di Lorenzo R. 1984. The caper culture in Italy. Acta Horticulturae: 167-172. https://doi.org/10.17660/actahortic.1984.144.21.
Batagelj V. 1988. Generalized Ward and related clustering problems. Classification and Related Methods of Data Analysis, 30: 67-74.
Beckmann J, Estivill X, and Antonarakis S. 2007. Copy number variants and genetic traits: closer to the resolution of phenotypic to genotypic variability. Nat. Rev. Genet. 8: 639-646. https://doi.org/10.1038/nrg2149.
Bourge, M., Brown, S. C., and Siljak-Yakovlev, S. (2018). Flow cytometry as tool in plant sciences, with emphasis on genome size and ploidy level assessment. Genet. Appl. 2, 1-12. https://hal.science/hal-03937019v1.
Caceres M, De Pace C, Mugnozza GS, Kotsonis P, Ceccarelli M, and Cionini PG. 1998. Genome size variations within Dasypyrum villosum: correlations with chromosomal traits, environmental factors and plant phenotypic characteristics and behaviour in reproduction. Theor. Appl. Genet. 96: 559-567. https://doi.org/10.1007/s001220050774.
Cardoso FC, Berri RA, Lucca G, Borges EN, and Mattos VLD. 2023. Normality tests: a study of residuals obtained on time series tendency modeling. Exacta. https://doi.org/10.5585/2023.22928.
Carr GD. 1978. Chromosome numbers of Hawaiian flowering plants and the significance of cytology in selected taxa. Am. J. Bot. 65: 236-242. https://doi.org/10.1002/j.1537-2197.1978.tb06061.x.
Carra A, Sajeva M, Abbate L, Siragusa M, Sottile F, and Carimi F. 2012. In vitro plant regeneration of caper (Capparis spinosa L.) from floral explants and genetic stability of regenerants. Plant Cell Tiss. Org. Cult. 109: 373-381. https://doi.org/10.1007/s11240-011-0102-9.
Carta A, Bedini G, and Peruzzi L. 2018. Unscrambling phylogenetic effects and ecological determinants of chromosome number in major angiosperm clades. Sci. Rep. 8: 14258. https://doi.org/10.1038/s41598-018-32515-x.
Cedillo-Cortezano M, Martinez-Cuevas LR, López JAM, Barrera López IL, Escutia-Perez S, and Petricevich VL. 2024. Use of medicinal plants in the process of wound healing: a literature review. Pharmaceuticals, 17(3): 303. https://doi.org/10.3390/ph17030303.
Chedraoui S, Abi-Rizk A, El-Beyrouthy M, Chalak L, Ouaini N, and Rajjou L. 2017. Capparis spinosa L. in A systematic review: A xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front. Plant Sci. 8. https://doi.org/10.3389/fpls.2017.01845.
Cincotta F, Merlino M, Verzera A, Gugliandolo E, and Condurso C. 2022. Innovative process for dried caper (Capparis spinosa L.) powder production. Foods 11. https://doi.org/10.3390/foods11233765.
Condurso C, Mazzaglia A, Tripodi G, Cincotta F, Dima G, Maria Lanza C, and Verzera A. 2016. Sensory analysis and head-space aroma volatiles for the characterization of capers from different geographic origin. J. Essent. Oil Res., 28(3): 185-192. https://doi.org/10.1080/10412905.2015.1113205.
Doležel J, and Bartoš J. 2005. Plant DNA flow cytometry and estimation of nuclear genome size. Ann. Bot. 95: 99-110. https://doi.org/10.1093/aob/mci005.
Doležel J, Bartos ., Voglmayr H, and Greilhuber J. 2003. Nuclear DNA content and genome size of trout and human. Cytometry. A 51: 127-128; author reply 129. https://doi.org/10.1002/cyto.a.10013.
Doležel J, Greilhuber J, and Suda J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2: 2233-2244. https://doi.org/10.1038/nprot.2007.310.
Doležel J, Sgorbati S, and Lucretti S. 1992. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 85: 625-631. https://doi.org/10.1111/j.1399-3054.1992.tb04764.x.
Ellul P, Boscaiu M, Vicente O, Moreno V, and Rosselló JA. 2002. Intra- and inter-specific variation in DNA content in Cistus (Cistaceae). Ann. Bot. 90: 345-351. https://doi.org/10.1093/aob/mcf194.
Firoozi N, Karimzadeh G, Sabet MS, and Sayadi V. 2022. Intraspecific karyomorphological and genome size variations of in vitro embryo derived Iranian endemic Asafoetida (Ferula assa-foetida L., Apiaceae). Caryologia 75: 111-121. https://doi.org/10.36253/caryologia-1721.
Gao Y, Zhao G, Xu Y, Hao Y, Zhao T, Jia L, and Chen Z. 2024. Karyotype analysis and genome size estimation of Sapindus mukorossi Gaertn. an economical important tree species in China. Bot. Lett. 171(12): 116-124. https://doi.org/10.1080/23818107.2023.2244179.
Garbari F, Bedini G, and Peruzzi L. 2012. Chromosome numbers of the Italian flora. From the Caryologia foundation to present. Caryologia 65: 62-71. https://doi.org/10.1080/00087114.2012.678090.
Goldblatt P. and Johnson ED. 1979. Index to plant chromosome numbers. Missouri Botanical Garden. Inc. Ann Arbour, Michigan. Available at: http://www.tropicos.org/Project/IPCN.
Gregory TR. 2005. Genome size evolution in animals. In the evolution of the genome. Academic Press. Pp. 3-87. https://doi.org/10.1016/B978-012301463-4/50003-6.
Greilhuber J, Doležel J, Lysák MA, Bennett MD. 2005. The origin, evolution and proposed stabilization of the terms ‘genome size’ and ‘C-value’ to describe nuclear DNA contents. Annals of Botany, 95(1): 255-260. https://doi.org/10.1093/aob/mci019.
Guerra M. 2012. Cytotaxonomy: The end of childhood. Plant Biosyst. 146: 703-710. https://doi.org/10.1080/11263504.2012.717973.
Gupta RC, and Gill BS. 1981. In chromosome number reports LXXI. Taxon 30: 514. http://www.jstor.org/stable/1220167.
Hamidi F, Karimzadeh G, Rashidi Monfared S, and Salehi M. 2018. Assessment of Iranian endemic Artemisia khorassanica: Karyological, genome size, and gene expressions involved in artemisinin production. Turk. J. Biol. 42: 322-333. https://doi.org/10.3906/biy-1802-86.
Harpke D, Carta A, Tomović G, Randelović V, Randelović N, Blattner FR, and Peruzzi L. 2015. Phylogeny, karyotype evolution and taxonomy of Crocus series Verni (Iridaceae). Plant Syst. Evol. 301: 309-325. https://doi.org/10.1007/s00606-014-1074-0.
Hinkelmann K. 2012. Design and Analysis of Experiments. John Wiley & Sons. https://doi.org/10.1002/9781118147634
Honarmand SJ, Nosratti I, Nazari K, and Heidari H. 2016. Factors affecting the seed germination and seedling emergence of muskweed (Myagrum perfoliatum). Weed Biol. Manag. 16: 186-193. https://doi.org/10.1111/wbm.12110.
Jang TS. and Weiss-Schneeweiss H. 2018. Chromosome numbers and polyploidy events in Korean non-commelinids monocots: A contribution to plant systematics. Korean J. Plant Taxon. 48: 260-277. https://doi.org/10.11110/kjpt.2018.48.4.260.
Javadian N, Karimzadeh G, Sharifi M, Moieni A, and Behmanesh M. 2017. In vitro polyploidy induction: changes in morphology, podophyllotoxin biosynthesis, and expression of the related genes in Linum album (Linaceae). Planta 245: 1165-1178. https://doi.org/10.1007/s00425-017-2671-2.
Kamel W, Abd El-Ghani MM, and El-Bous M. 2009. Taxonomic study of Capparaceae from Egypt: revisited. African J. Pl. Sci. Biotech, 3: 27-35.
Karimzadeh G, Danesh-Gilevaei M., and Aghaalikhani M. 2011. Karyotypic and nuclear DNA variations in Lathyrus sativus (Fabaceae). Caryologia 64: 42-54. https://doi.org/10.1080/00087114.2011.10589763.
Khakshour A, Karimzadeh G, Sabet MS, and Sayadi V. 2024. Karyomorphological and genome size variation in Iranian endemic populations of coriander (Coriandrum sativum L.). Cytologia 89: 21-27. https://doi.org/10.1508/cytologia.89.21.
Khatoon S, and Ali SI. 1993. Chromosome atlas of the angiosperms of Pakistan. Karachi Univ. Karachi vii, 232 p., ISBN 1104765435.
Kocjan D, Dolenc Koce J, Etl F, and Dermastia M. 2022. Genome size of life forms of Araceae-A new piece in the C-value puzzle. Plants (Basel, Switzerland) 11. https://doi.org/10.3390/plants11030334/
Kondrashov FA, Rogozin IB, Wolf YI, and Koonin EV. 2002. Selection in the evolution of gene duplications. Genome Biol. 3: 1-9. https://doi.org/10.1186/gb-2002-3-2-research0008.
Kula A. 1999. Cytogenetic studies in the cultivated form of Bromus carinatus (Poaceae). W. Szafer Institute of Botany, Polish Academy of Sciences. 101-106.
Levin DA. 2002. The role of chromosomal change in plant evolution. Oxford University Press, USA.
Loureiro J, Rodriguez E, Doležel J, and Santos C. 2007. Two new nuclear isolation buffers for plant DNA flow cytometry: A test with 37 species. Ann. Bot. 100: 875-888. https://doi.org/10.1093/aob/mcm152.
Magulaev AJ. 1979. The chromosome numbers of flowering plants in the northern Caucasus. part 3. Flora north Caucasus Quest. Its Hist. 3: 101-106.
Mahdavi S, and Karimzadeh G. 2010. Karyological and nuclear dna content variation in some Iranian endemic Thymus species (Lamiaceae). J. Agric. Sci. Technol. 12: 447-458.
Mahmoudi S, and Mirzaghaderi G. 2023. Tools for Drawing Informative Idiograms Methods in Molecular Biology, (Springer). 515-527. https://doi.org/10.1007/978-1-0716-3226-0_31.
Marir EMA. 2024. Propagation of medicinal capers (Capparis spinosa L.) and production of some medicinal secondary metabolic compounds using plant tissue culture technology. Euphrates J. Agric. Sci. 16.
Matthäus B, and Özcan M. 2005. Glucosinolates and fatty acid, sterol, and tocopherol composition of seed oils from Capparis spinosa var. spinosa and Capparis ovata Desf. var. canescens (Coss.) Heywood. J. Agric. Food Chem. 53: 7136-7141. https://doi.org/10.1021/jf051019u.
Mayrose I, and Lysak MA. 2021. The evolution of chromosome numbers: mechanistic models and experimental approaches. Genome Biol. Evol. 13. https://doi.org/10.1093/gbe/evaa220.
Mehravi S, Karimzadeh G, Kordenaeej A, and Hanifei M. 2022a. Mixed-ploidy and dysploidy in Hypericum perforatum: A karyomorphological and genome size study. Plants 11(22): 3068. https://doi.org/10.3390/plants11223068.
Mehravi S, Ranjbar GA, Najafi-Zarrini H, Mirzaghaderi G, Hanifei M, Severn-Ellis AA, Edwards D, and Batley J. 2022b. Karyology and genome size analyses of Iranian endemic Pimpinella (Apiaceae) species. Front. Plant Sci. 13: 1-14. https://doi.org/10.3389/fpls.2022.898881.
Merlino M, Condurso C, Cincotta F, Nalbone L, Ziino G, and Verzera A. 2024. Essential oil emulsion from caper (Capparis spinosa L.) leaves: exploration of its antibacterial and antioxidant properties for possible application as a natural food preservative. Antioxidants 13(6) : 718. https://doi.org/10.3390/antiox13060718.
Mesquita AT, Braz GT, Shimizu GH, Machado RM, Romero-da Cruz MV, and Forni-Martins ER. 2024. Karyotype diversity and genome size in the Cyphomandra clade of Solanum L. (Solanaceae). Bot. J. Linn. Soc. https://doi.org/10.1093/botlinnean/boae047.
Moghaddasi MS. 2011. Caper (Capparis spp.) importance and medicinal usage. Adv. Environ. Biol. 5(5): 872-879.
Mohammadpour S, Karimzadeh G, and Ghaffari SM. 2022. Karyomorphology, genome size, and variation of antioxidant in twelve berry species from Iran. Caryologia 75: 133-148. https://doi.org/10.36253/CARYOLOGIA-1633.
Morales Valverde R. 1986. Taxonomia De Los Generos Thymus (Excluida De La Seccion Serpyllum) Y Thymbra En La Peninsula Iberica). CSIC – Real Jardin Botanico (RJB), Ruizia. Monografias del Jardin Botanico 3: 324 p. http://hdl.handle.net/10261/66682.
Morovati Z, Karimzadeh G, Naghavi MR, and Rashidi Monfared S. 2024. Chromosome, ploidy analysis, and flow cytometric genome size estimation of Datura stramonium and D. innoxia medicinal plant. Caryologia, 77(3): 53-61. https://doi.org/10.36253/caryologia-2768.
Nabavi SF, Maggi F, Daglia M, Habtemariam S, Rastrelli L, and Nabavi SM. 2016. Pharmacological effects of Capparis spinosa L. Phyther. Res. 30(11): 1733-1744. https://doi.org/10.1002/ptr.5684.
Naranjo CA, Ferrari MR, Palermo AM, and Poggio L. 1998. Karyotype, DNA content and meiotic behaviour in five South American species of Vicia (Fabaceae). Ann. Bot. 82: 757-764. https://doi.org/10.1006/anbo.1998.0744.
Ning H, Ao S, Fan Y, Fu J, and Xu C. 2018. Correlation analysis between the karyotypes and phenotypic traits of Chinese Cymbidium cultivars. Hortic. Environ. Biotechnol. 59: 93-103. https://doi.org/10.1007/s13580-018-0010-6.
Olmez Z, Gokturk A, and Gulcu S. 2006. Effects of cold stratification on germination rate and percentage of caper (Capparis ovata Desf.) seeds. J. Environ. Biol. 27(4): 667-670.
Osborne JW. 2010. Improving your data transformations: Applying the Box-Cox transformation. Pract. Assessment, Res. Eval. 15. https://doi.org/10.7275/qbpc-gk17.
Patwardhan D, Varshini SA, and Galoth L. 2022. Study of Chromosome. In Genetics Fundamentals Notes. Singapore: Springer Nature Singapore. pp 239-298. https://doi.org/10.1007/978-981-16-7041-1_5.
Pellicer J, Hidalgo O, Dodsworth S, and Leitch IJ. 2018. Genome size diversity and its impact on the evolution of land plants. Genes (Basel). 9(2): 88. https://doi.org/10.3390/genes9020088.
Peruzzi L and Altinordu,F. 2014. A proposal for a multivariate quantitative approach to infer karyological relationships among taxa. Comp. Cytogenet. 8: 337-349. https://doi.org/10.3897/CompCytogen.v8i4.8564.
Peruzzi L and Eroǧlu HE. 2013. Karyotype asymmetry: Again, how to measure and what to measure? Comp. Cytogenet. 7: 1-9. https://doi.org/10.3897/CompCytogen.v7i1.4431.
Peruzzi L, Carta A, and Altinordu F. 2017. Chromosome diversity and evolution in Allium (Allioideae, Amaryllidaceae). Plant Biosyst. 151: 212-220. https://doi.org/10.1080/11263504.2016.1149123.
Peruzzi L, Góralski G, Joachimiak AJ, and Bedini G. 2012. Does actually mean chromosome number increase with latitude in vascular plants? An answer from the comparison of Italian, Slovak and Polish floras. Comp. Cytogenet. 6: 371. https://doi.org/10.3897/CompCytogen.v6i4.3955.
Qi J, Liang W, Yunlin Z, Guiyan Y, Tianci T, Yingzi M, Zhenggang X. 2023. Methods for rapid seed germination of Broussonetia papyrifera. Pak. J. Bot. 55: 941-948. https://doi.org/10.30848/PJB2023-3(2).
Radmanesh P, Karimzadeh G, Kashkoli AB, and Heidarzadeh A. 2023. Study on phytochemical traits and improving seed germination methods of Iranian endemic populations of caper (Capparis spinosa L.) medicinal plant. Iran. J. Seed Sci. Res. 10: 41-52. (In Persian with English Abstract). https://doi.org/10.22124/jms.2023.23352.1729.
Rasekh SZ and Karimzadeh G. 2023. Chromosomal and genome size variations in opium poppy (Papaver somniferum L.) from Afghanistan. Caryologia, 76(4): 15-22. https://doi.org/10.36253/caryologia-1955.
Rock BN. 2016. The woods and flora of the Florida Keys : “Pinnatae” /. woods flora Florida Keys “Pinnatae” https://doi.org/10.5962/bhl.title.123255.
Runemark H. 1996. Mediterranean chromosome number reports 6 (590-678). Flora Mediterr. 6, 223-243.
Sakcali MS, Bahadir H, and Ozturk M. 2008. Eco-physiology of Capparis spinosa L.: A plant suitable for combating desertification. Pak. J. Bot, 40(4): 1481-1486. https://www.academia.edu/download/31556522/PJB40(4)1481.pdf.
Sandhu PS. 1989. SOCGI plant chromosome number reports 8. J. Cytol. Genet. 24, 179-183.
Sayadi V, Karimzadeh G, Naghavi MR, and Rashidi Monfared S. 2022. Interspecific genome size variation of Iranian endemic Allium species (Amaryllidaceae). Cytologia (Tokyo). 87: 335-338. https://doi.org/10.1508/cytologia.87.335.
Shahrajabian MH, Sun W, and Cheng Q. 2021. Plant of the millennium, caper (Capparis spinosa L.), chemical composition and medicinal uses. Bull. Natl. Res. Cent. 45. https://doi.org/10.1186/s42269-021-00592-0.
Shariat A, Karimzadeh G, and Assareh MH. 2013. Karyology of Iranian endemic Satureja (Lamiaceae) species. Cytologia (Tokyo). 78: 305-312. https://doi.org/10.1508/cytologia.78.305.
Sharma A. (1968). Chromosome number reports of plants. In Annual Report, Cytogenetics Laboratory, Department of Botany, University of Calcutta. Res. Bull. 2: 38-48.
Siljak-YakovlevS and Peruzzi L. 2012. Cytogenetic characterization of endemics: Past and future. Plant Biosyst. 146, 694-702. https://doi.org/10.1080/11263504.2012.716796.
Singh RJ. 2016. Plant Cytogenetics (3rd ed.). CRC Press. 548 p. https://doi.org/10.1201/9781315374611.
Singhal VK and Gill BS. 1984. SOCGI plant chromosome number reports II. J. Cytol. Genet 19: 115-117.
Sliwinska E. 2018. Flow cytometry-a modern method for exploring genome size and nuclear DNA synthesis in horticultural and medicinal plant species. Folia Hortic. 30: 103-128. https://doi.org/10.2478/fhort-2018-0011.
Stace CA. 2000. Cytology and cytogenetics as a fundamental taxonomic resource for the 20th and 21st centuries. Taxon 49: 451-477. doi.org/10.2307/1224344.
Stebbins GL. 1950. Variation and Evolution in Plants. Columbia University Press, USA.
Stebbins GL. 1971. Chromosomal Evolution in Higher Plants. Edward Arnold Ltd, UK.
Subramanian D. and Pondmudi R. 1987. Cytotaxonomical Studies of South Indian Scrophulariaceae. Cytologia (Tokyo). 52: 529-541. https://doi.org/10.1508/cytologia.52.529.
Sundarrajan P and Bhagtaney L. 2023. Traditional Medicinal Plants as Bioresources in Health Security. Apple Academic Press. https://doi.org/10.1201/9781003352983-3.
Swift HH. 1950. The constancy of desoxyribose nucleic acid in plant nuclei. Proceedings of the National Academy of Sciences, Washington 36: 643-654. https://doi.org/10.1073/pnas.36.11.643.
Tarkesh Esfahani S, Karimzadeh G, and Naghavi MR. 2016. 2C DNA value of Persian poppy (Papaver bracteatum Lindl.) medicinal plant as revealed by flow cytometry analysis; a quick effective criteria for distinguishing unidentified Papaver species. International Journal of Advanced Biotechnology and Research, 7(2): 573-578. http://www.bipublication.com.
Tarkesh Esfahani S, Karimzadeh G, and Naghavi MR. 2020. In vitro polyploidy induction in persian poppy (Papaver bracteatum Lindl.). Caryologia 73: 133-144. https://doi.org/10.13128/caryologia-169.
Tavan M, Mirjalili MH, and Karimzadeh G. 2015. In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tiss. Org. Cult.122: 573-583. https://doi.org/10.1007/s11240-015-0789-0.
Tkach N and Röser M. 2024. Genome sizes of grasses (Poaceae), chromosomal evolution, paleogenomics and the ancestral grass karyotype (AGK). https://doi.org/10.21203/rs.3.rs-3914153/v1.
Tlili N, Mejri H, Anouer F, Saadaoui E, Khaldi A, and Nasri N. 2015. Phenolic profile and antioxidant activity of Capparis spinosa seeds harvested from different wild habitats. Industrial Crops and Products, 76: 930-935. https://doi.org/10.1016/j.indcrop.2015.07.040.
Vimala Y, Lavania S, and Lavania UC. 2021. Chromosome change and karyotype differentiation-implications in speciation and plant systematics. Nucl. 64: 33-54. https://doi.org/10.1007/s13237-020-00343-y.
Wang LJ, Gao MD, Sheng MY, and Yin J. 2020. Cluster analysis of karyotype similarity coefficients in Epimedium (Berberidaceae): Insights in the systematics and evolution. PhytoKeys 161: 11-26. https://doi.org/10.3897/PHYTOKEYS.161.51046.
Wang L, Fan L, Zhao Z, Zhang Z, Jiang L, Chai M, and Tian C. 2022. The Capparis spinosa var. herbacea genome provides the first genomic instrument for a diversity and evolution study of the Capparaceae family. Gigascience 11: 1-14. https://doi.org/10.1093/gigascience/giac106.
Weigel D and Nordborg M. 2015. Population genomics for understanding adaptation in wild plant species. Annu. Rev. Genet. 49(1): 315-338. https://doi.org/10.1146/annurev-genet-120213-092110.
Winterfeld G, Ley A, Hoffmann MH, Paule J, and Röser M. 2020. Dysploidy and polyploidy trigger strong variation of chromosome numbers in the prayer-plant family (Marantaceae). Plant Syst. Evol. 306: 1-17. https://doi.org/10.1007/s00606-020-01663-x.
Winterfeld G, Schneider J, Becher H, Dickie J, and Röser M. 2015. Karyosystematics of the Australasian stipoid grass Austrostipa and related genera: chromosome sizes, ploidy, chromosome base numbers and phylogeny. Aust. Syst. Bot. 28: 145-159. https://doi.org/10.1071/sb14029.
Xiaofeng G and Richman MB. 1995. On the application of cluster analysis to growing season precipitation data in North America east of the Rockies. J. Clim. 8: 897-931. https://doi.org/10.1175/1520-0442(1995)008<0897:otaoca>2.0.co;2.
Yari A, Karimzadeh G, Rashidi Monfared ., and Sayadi S. 2024. Mixed-ploidy in Iranian endemic Cymbopogon olivieri (Boiss.) Bor: A chromosomal and holoploid genome size study. Cytologia, 89 (2): 127-131. https://doi.org/10.1508/cytologia.89.127.
Yeshitila M, Gedebo A, Tesfaye B, Demissie H, and Olango TM. 2023. Multivariate analysis for yield and yield-related traits of amaranth genotypes from Ethiopia. Heliyon. 9: 100184. https://doi.org/10.1016/j.heliyon.2023.e18207.
Zarabizadeh H, Karimzadeh G, Rashidi Monfared S, and Tarkesh Esfahani S. 2022. Karyomorphology, ploidy analysis, and flow cytometric genome size estimation of Medicago monantha populations. Turk. J. Botany 46: 50-61. https://doi.org/10.3906/bot-2105-22.
Zare Teymoori S, Karimzadeh G, and Shariat A. 2021. Chromosomal and genome size diversity in savory (Satureja spp.) medicinal plant. Iranian Journal of Rangelands and Forests Plant Breeding and Genetic Research, 29(2): 236-250. https://doi.org/10.22092/ijrfpbgr.2021.354486.1383. (In Persian with English abstract).
Zarei M, Seyedi N, Maghsoudi S, Nejad MS, and Sheibani H. 2021. Green synthesis of Agnanoparticles on the modified graphene oxide using Capparis spinosa fruit extract for catalytic reduction of organic dyes. Inorg. Chem. Commun. 123. https://doi.org/10.1016/j.inoche.2020.108327.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Ghasem Karimzadeh, Parviz Radmanesh

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Copyright on any open access article in a journal published byCaryologia is retained by the author(s).
- Authors grant Caryologia a license to publish the article and identify itself as the original publisher.
- Authors also grant any third party the right to use the article freely as long as its integrity is maintained and its original authors, citation details and publisher are identified.
- The Creative Commons Attribution License 4.0 formalizes these and other terms and conditions of publishing articles.
- In accordance with our Open Data policy, the Creative Commons CC0 1.0 Public Domain Dedication waiver applies to all published data in Caryologia open access articles.