Exploring the Potential of Dacryodes Edulis Leaf Extract as Natural Colourant on Polyamide Fabrics: Extraction, Characterization and Application
Published 2024-06-20
Keywords
- Natural dye,
- Extraction,
- Characterization,
- Response surface methodology,
- Dacryodes edulis
- leaf,
- polyamide,
- fabrics ...More
How to Cite
Copyright (c) 2024 Poro Clark, Johnson Otutu, Augustine Kanayochukwu Asiagwu, Gloria Ndukwe

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
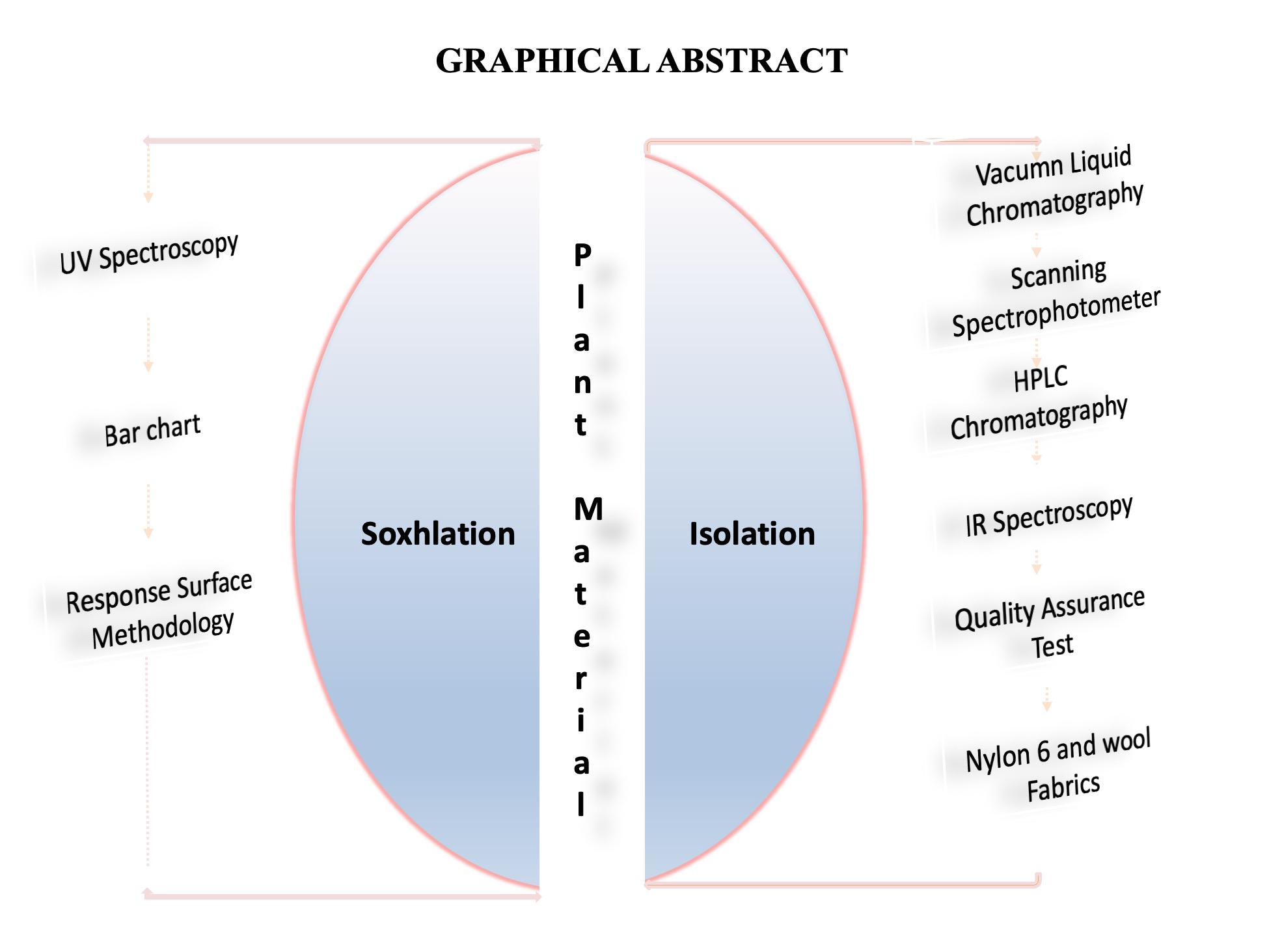
There is a growing demand for sustainable and eco-friendly alternatives in the textile industry, particularly in search of natural colourants derived from plants. This research study investigates the extraction and characterization of natural colourant from the leaf of D. edulis and explores its application to polyamide fabrics. Laboratory experiments, such as solvent extraction was performed to obtain the colourant. The extraction process was optimized using Response Surface Methodology-Central Composite Design (RSM). The dye was isolated and characterized using vacuum liquid chromatography, UV-Vis spectrophotometry, high-performance liquid chromatography and Fourier-transform infrared spectroscopy. Physical properties such as light fastness, perspiration fastness, rubbing fastness and wash fastness were assessed to determine the durability and stability of the natural colourant on the fabrics. The results indicated that the optimal conditions for dye extraction are 65.9 ℃ and 2 hours, providing feasible parameters for replication. Examination of the natural dye obtained from the isolated fraction, revealed the presence of carbon-carbon double bonds (C=C), carbonyl (C=O), ester (-COO), aldehyde (CHO) and hydroxyl (-OH) groups; with rutin, isoquercetin and tannic acid as its major compounds. The isolated dye exhibited an absorption peak at 403 nm. The ratings for the treated fabrics varied from fair to good and very good. The light fastness ranged from 4 to 6, perspiration fastness and wash fastness ranged from 2-4, and rubbing fastness ranged from 3-5. On the other hand, the untreated fabrics had ratings, with a range of 2-5 for light fastness, 2-4 for perspiration fastness and wash fastness and 1-4 for rubbing fastness, it was also observed that the colour strength values of mordanted fabrics were deeper than those of the unmordanted fabrics. These results indicate that the dye obtained from D. edulis leaf has considerable potential as a source of natural dyes. These findings contribute valuable insights into the extraction, characterization and application of natural colourants, promoting a shift towards more sustainable and eco-friendly practices in the textile industry.
References
- S. Ratnapandian, S. Islam, S.M. Fergusson, L. Wang, R. Padhye, Colouration of cotton by combining natural colourants and bio-polysaccharide, J. Text Inst., 2013, 102(12), 1269-1276. https://doi.org/10.1080/00405000.2013.797143
- I.A. Samant, D.K. Gaikwad, Optimization of natural dye extraction from coconut husk, J Exp. Biol. Agric. Sci., 2020, 8(1), 54-62. https://doi.org/10.18006/2020.8(1).54.62
- D.R.B. Lekshmi, D. Ravi, Extraction of natural dyes from selected plants sources and its application in fabrics, Int. J. Text. Fash. Technol., 2013, 3(2), 2250-2378.
- M.A. Ezeokonkwo, S.N. Okafor, E.U. Godwin-Nwakwasi, Preliminary characterization of some natural dyes, Am. J. Pure Appl Chem., 2018, 12(7), 55-61. https://doi.org/10.5897/ajpac2018.0767
- F. Asian, New natural dyes extracted by ultrasonic and soxhlet method: Effect on dye-sensitized solar cell photovoltaic performance, Opt. Quant. Electron., 2024, 56, 1-22.
- V.P. Careaga, A.B. Guerrero, G. Siracusano, M.S. Maier, High performance liquid chromatography as a micro-destructive technique for the identification of anthraquinone red dyestuffs in cultural heritage objects, Chem. Teach. Int., 2023, 5(1), 1-9.
- K. Son, D. Yoo, Y. Shin, Developing an ecofriendly indigo dyeing system by using Baker’s yeast (Saccharomyces cerevisiae): Some variables related to PH control and scale-up, Fibers Polym., 2020, 21, 1790-1797.
- L. Loredana, A. Giuseppina, N. Filomena, F. Florinda, D. Marisa, A. Donatella, Biochemical, antioxidant properties and antimicrobial activity of different onion varieties in the mediterranean area. J. Food Meas. Charact., 2019, 13, 1232-1241.
- B. Wei, Q. Chen, G. Chen, R. Tang, J. Zheng, Adsorption properties of lac dye on wool, silk and nylon. J. Chem., 2013, 13, 1-6.
- M.S. Kiakhani, Eco-friendly dyeing of wool and nylon using madder as a natural dye: Kinetic and adsorption isotherm studies. IJEST., 2015, 12, 2363-2370.
- K.K. Ajibesin, Dacryodes edulis (G.don) HJ Lam: A review on its medicinal, phytochemical and economical properties, Res. J. Med. Plant., 2011, 5(1), 32-41. https://doi.org/10.3923/rjmp.2011.32.41
- C.S. Kumar, S. Dhinakaran, Extraction and application of natural dyes from orange peel and lemon peel on cotton fabrics, Int. Res. J. Eng.Tech., 2017, 4(5), 237-238.
- A. Vasileiadou, I. Karapanagiotis, A. Zotou, A chromatographic investigation on ageing of natural dyes in silk under UV light, Archaeol. Anthropol Sci, 2022, 14, 21-32.
- F. Vladimir, P. Martin, V. Michaela, L. Juraj, Application of response surface methodology (RSM) for optimization of zinc extraction from anaerobic sewage sludge, Environ. Eng & Manag J., 2018, 7, 1685-1692. https://doi.org/10.30638/eemj.2018.167
- G.I. Ndukwe, S.Y. Garba, E.A. Adelakun, Activity-guided isolation and antimicrobial assay of a
- flavonol from Mitracarpus verticillatus (Schumach. & Thonn.) Vatke, IOSR-JAC., 2016, 9(9), 118-131. https://doi.org/10.9790/5736-090902118131
- P.A. Paranagama, Vacuum liquid chromatography and gel permeation chromatography in natural product research, Nat. Work. Sep. Tech. Nat. Prod. Res., 2016, 95-98.
- G.I. Ndukwe, A. Oluah, G.K. Fekarurhobo, Isolation of an isoflavonoid and a terpenoid from the heartwood of Baphia nitida Lodd. (camwood), Ovidius Univ. Ann. Chem., 2020, 31(1), 5-8. https://doi.org/10.2478/auoc-2020-0002
- I.R. Jack, P.D. Clark, G.I. Ndukwe, Evaluation of phytochemical, antimicrobial and antioxidant capacities of Pennisetum purpureum (Schumach) extracts, Chem. Sci Int. J. 2020, 29(4), 1-14. https://doi.org/10.9734/csji/2020/v29i430170
- P.D. Clark, E.A. Omo-Udoyo, Comparative assessment on antioxidant and phytochemical of Trichillia Monadelpha (Thonn) J.J. De Wilde (Meliaceae) plant extracts, Chem. Sci Int. J. 2021, 30(10), 37-38. https://doi.org/10.9734/csji/2021/v30i1030257
- P.D. Clark, J.O. Otutu, G.I. Ndukwe, C.A. Idibie, Investigating the feasibility of utilizing Pennisetum purpureum leaves waste as a sustainable dye: Extraction, characterization and application on textile, Scien. Afric., 2023, 22(3), 231-246.
- N. Baaka, M.T. Ben, W. Hadder, S. Hammami, M.F. Mhenni, Extraction of natural dye from waste wine industry: Optimization survey based on central composite design model, Fibers Polym., 2015, 16(1), 38-45. https://doi.org/10.1007/s12221-015-0038-5.
- M.A.M. Al-Alwani, A.B. Mohamad, A.A.H. Kadhum, N.A. Ludin, Effect of solvents on the extraction of natural pigments and adsorption onto TiO2 for dye-sensitized solar cell applications, Spectrochmica Acta Part A Mol Biomol Spectrosc., 2015, 138, 130-137. https://doi.org/10.1016/j.saa.2014.11.018
- H.Z. Suwari, Y. kotta, L. Buang, Optimization of soxhlet extraction and physiochemical analysis of crop oil from seed kernel of Feun kase (Thevetia peruviana), AIP Conf Proc., 2017, 1-5. https://doi.org/10.1063/1.5015998.
- P.R. Carmen, M.R. Juan, R. Encarnacion, C. Eulogio, Optimization with response surface methodology of microwave-assisted conversion of xylose to furfural. Mol., 2020, 25(16), 3574-3579. https://doi.org/10.3390/molecules25163574.
- B. Ayoub, K. Alireza, K. Hossein, D.A. Seyed, N. Dariush, Modeling of azo dyes absorption on magnetic NiFe2O4 / RGO nanocomposite using response surface methodology., J. Environ. Health Sci. Eng., 2013, 17, 931-947. https://doi.org/10.1007/s40201-019-00409-3.
- A. Hassani, R.D.C. Soltani, M. Kiransan, S. Karaca, C. Karaca, A. Khataee, Ultrasound-assisted adsorption of textile dyes using modified nanoclay: Central composite design optimization, Korean J. Chem., 2015, 33, 178-188. https://doi.org/10.1007/s11814-015-0106-y.
- B.S. Aponjolosun, T.R. Fasola, Phytochemical, antimicrobial and toxicity assessment of Dacryodes edulis (G. Don.) H. J. Lam leaf extracts, Afr. J. Biomed. Res., 2022, 4(4), 113-122. https://doi.org/10.26538/tjnpr/v4i4.1
- L. Liu, L. Chen, A.M. Abbasi, Z. Wang, D. Li, Y. Shen, Optimization of extraction of polyphenols from Sorghum moench using response surface methodology, and determination of their antioxidant activities. Trop. J. Pharm. Res., 2018, 17(4), 619-626. https://doi.org/10.4314/tjpr.v17i4.8
- H.S. Yim, F.Y. Chye, V. Rao, J.Y. Low, P. Matanjun, S.E. How, C.W. Ho, Optimisation of extraction time and temperature on antioxidant activity of Schizophyllum commune aqueous extract using response surface methodology, J. Food Technol., 2013, 50(2), 275-283. https://doi.org/10.1007/s13197-011-0349-5.
- L. Janani, D. Winifred, Suitability of dyes from mulberry and coffee leaves on silk fabrics using eco-friendly mordants, IJSRP., 2013, 3(11), 1-4.
- C.S. Jamieson, J. Misa, Y. Tang, J.M. Billingsley, Biosynthesis and synthetic biology of psychoactive natural products, Chem. Soc. Rev., 2021, 50, 6950-7008. https://doi.org/10.1039/d1cs00065a.
- S.B. Christensen, Natural products that changed the society, Biomedicines., 2021, 9(5), 472-479. https://doi.org/10.3390/biomedicines9050472.
- L. Barleben, S. Panjikar, M. Ruppert, J. Koepke, J. Stockigt, Molecular architecture of strictosidine glucosidase: The gateway to the biosynthesis of the monoterpenoid indole alkaloid family, The Plant Cell., 2007, 19(9), 2886-2897. https://doi.org/10.1105/tpc.106.045682.
- A.M. Engida, N.S. Kasim, Y.A. Tsigie, S. Ismadji, L.H. Huynh, Y. Ju, Extraction, identification and quantitative HPLC analysis of flavonoids from Sarang semut (Myrmecodia pendan), Ind. Crops. Prod., 2013, 41, 392-396. https://doi.org/10.1016/j.indcrop.2012.04.043
- M.Z.M. Salem, I.H.M. Ibrahim, H.M. Ali, H.M. Helmy, Assessment of the use of natural extracted dyes and pancreatin enzyme for dyeing of four natural textiles, Processes., 2020, 8(1), 59-64. https://doi.org/10.3390/pr8010059.
- C. Karpagasundari, S. Kulothungan, Analysis of bioactive compounds in Physalis minima leaves using GC MS, HPLC, UV-VIS and FTIR techniques, J. Pharmacogn. Phytochem., 2014, 3(4), 196-201.
- T.K. Patle, K. Shrivas, R. Kurrey, S. Upadhyay, R. Jangde, R. Chauhan, Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV-vis and FTIR Spectroscopy, Spectrochimica Acta Part A Mol. Biomol.Spectrosc., 2020, 242, 118-120. https://doi.org/10.1016/j.saa.2020.118717.
- M.E.S. Mirghani, N.A. Kabbashi, I.Y. Qudsieh, F.A. Elfaki, A new method for the determination of toxic dye using FTIR Spectroscopy, IIUM Eng. J., 2008, 9(2), 27-38. https://doi.org/10.31436/iiumej.v9i2.98.
- N. Sofyan, A. Ridhova, K.R.O. Pramono, A.H. Yuwono, A. Udhiarto, Visible light absorption and photo-sensitizing characteristics of natural dye extracted from Mangosteen pericarps using different solvents, Int. J. Adv Sci Eng. Inf. Technol., 2018, 8(5), 2059-2064. https://doi.org/10.18517/ijaseit.8.5.3499.
- A. Talukdar, Extraction, isolation and characterization of natural dye from kathanda (Tabernaemontanum, divericata) and vaola (Mangifera macrophilla), Int. J. Res Technol., 2018, 11, 42-45.
- M.T. Uddin, M.A. Razzag, A.H. Quadery, M.J. Chowdhury, A. Mizan, M. Raihan, F. Ahmad, Extraction of dye from natural source (LAC) & its application on leather, ASRJETS., 2017, 34(1), 1-7.
- A. Ado, H. Yahaya, A.A. Kwalli, R.S. Abdulkadir, Dyeing of textiles with eco-friendly natural dyes: A review, Int. J. Environ Moni. Protect., 2015, 1(5), 76-81.
- M.A.I. Salem, M.I. Marzouk, H.M. Mashaly, Synthesis of pharmacological dyes and their application on synthetic fabrics, Color Technol., 131(4), 288-296. https://doi.org/10.1111/cote.12155
- R. Mongkholrattanasit, J. Krystufek, J. Wiener, J. Studnickova, Properties of wool and cotton fabrics dyed with Eucalptus, tannin and flavonoids, Fibres Text. East. Eur., 2011, 19(2), 90-95.
- S.N. Okonkwo, C.B.C. Ohanuzue, G.C. Oneugbu, H.C. Obasi, O.O. Nnorom, Extraction of natural dyes from Whitfieldia lateritia plant and its application on cotton fabric, JTESE., 2019, 9, 392-397. https://doi.org/10.4172/2165
- R. Mongkholrattanasit, J. Krystufek, J. Wiener, Dyeing and fastness properties of natural dyes extracted from Eucalyptus leaves using padding techniques, Fibers Polym., 2010, 11, 346-350. https://doi.org/10.1007/s12221-010-0346-8
- D. Malomo, S. Abimbade, A.K. Olwaseun, O. Eghareba, Likely mechanism of dye adhesion on fabrics. Proceedings of 62nd ISERD International Conference, Boston, USA, 2017, 9-15.
- R. Mongkholrattanasit, J. Krystufek, J. Wiener, J. Studnickova, Properties of wool and cotton fabrics dyed with Eucalptus, tannin and flavonoids, Fibres Text. East. Eur., 2011, 19(2), 90-95.
- R. Prabhavathi, A.S. Devi, D. Anitha, A. Padma, An improving perspiration fastness of annatto natural dye on the cotton fabric, Int. J. Educ Sci Res., 8(6), 13-22. https://doi.org/10.24247/ijesrdec20182
- J. Wang, Y. Gao, L. Zhu, X. Gu, H. Dou, L. Pei, Dyeing property and adsorption kinetics of reactive dyes for cotton textiles in salt-free NIN-aqueous dyeing systems, Polym., 2018, 10I(9), 1030-1043.





