Published 2023-04-20
Keywords
- Emulsion,
- Produced water,
- Demulsifier,
- Demulsification,
- Oil-in-water content (OiW)
- Demulsifier OA-KX ...More
How to Cite
Copyright (c) 2023 Habineswaran Rajan, Nur’aini Raman Yusuf, Dzeti Farhah Mohsim, Nor Hadhirah Bt Halim

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
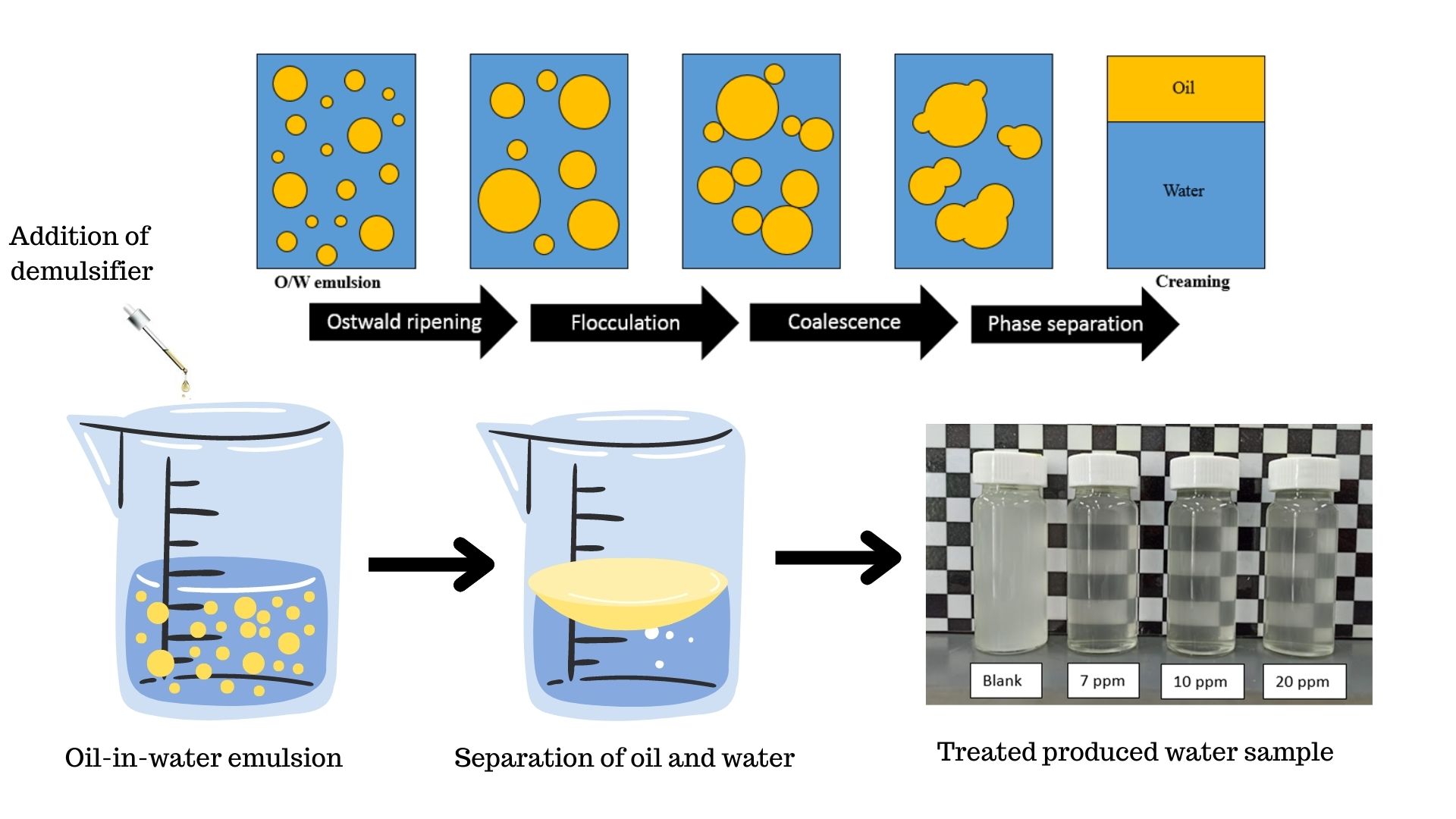
Produced water, also known as oily wastewater, is one of the major wastes in the oil and gas industry. During the hydrocarbon production, formation of emulsion takes place such as oil-in-water emulsion which has a huge financial effect on the sector. Oil and gas industry seeks highly effective and reasonable demulsifying chemicals to separate the oil-in-water emulsions into water and crude oil. Thus, in this publication, resin alkoxylate, cationic polyamine, cationic surfactant and ethylene oxide/propylene oxide (EO/PO) block copolymers are utilized to resolve the oil-in-water emulsion from a gas condensate field. According to the findings of preliminary screening, a unique demulsifier DB was formulated by incorporating resin alkoxylate and cationic surfactant at an optimal weight percentage ratio. Demulsification efficiency (De) of 96 % based on measurement of turbidity was attained after treating the oil-in-water (O/W) emulsion with demulsifier DB at a dosage of 7 ppm. To determine the demulsifier's efficiency further, the oil-in-water content (OiW) of the produced water was evaluated after the treatment with demulsifier DB. Oil removal efficiency (ORe) of 90% was achieved as the formulated demulsifier DB reduced the oil-in-water content (OiW) of O/W emulsion from 1008.3 ppm to 97.1 ppm within 15 minutes at the dosage of 7 ppm. Furthermore, interfacial tension (IFT) and Turbiscan analysis were performed to further study the demulsification process of blank sample and the addition of the demulsifier DB at the optimized dosage of 7 ppm. At demulsifier DB dosage of 7 ppm, the interfacial tension between oil and water reduced significantly compared to blank sample from 24.98 mN/m to 9.38 mN/m. The produced water sample after treatment with 7 ppm of demulsifier DB resulted in a significant increase of Turbiscan Stability Index (TSI) value of 8 which indicates the rate at which the separation of oil and water occurred. The attained results of IFT and Turbiscan analysis further validate that mixed surfactant system is more efficient than single surfactant system. By combining surfactants with different functional groups, mixed surfactant systems can exhibit greater surface activity than single surfactants.
References
- Abdulredha, M. M., Siti Aslina, H., & Luqman, C. A. (2020). Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arabian Journal of Chemistry, 13(1), 3403–3428. https://doi.org/10.1016/j.arabjc.2018.11.014
- Acosta, M., Reyes, L. H., Cruz, J. C., & Pradilla, D. (2020). Demulsification of Colombian Heavy Crude Oil (W/O) Emulsions: Insights into the Instability Mechanisms, Chemical Structure, and Performance of Different Commercial Demulsifiers. Energy & Fuels, 34(5), 5665–5678. https://doi.org/10.1021/acs.energyfuels.0c00313
- Aswathanarayan, J. B., & Vittal, R. R. (2019). Nanoemulsions and Their Potential Applications in Food Industry. Frontiers in Sustainable Food Systems, 3. https://doi.org/10.3389/fsufs.2019.00095
- Auflem, I. H. (2002). Influence of asphaltene aggregation and pressure on crude oil emulsion stability. Norges teknisk-naturvitenskapelige universitet, Trondheim.
- Celia, C., Trapasso, E., Cosco, D., Paolino, D., & Fresta, M. (2009). Turbiscan Lab® Expert analysis of the stability of ethosomes® and ultradeformable liposomes containing a bilayer fluidizing agent. Colloids and Surfaces B: Biointerfaces, 72(1), 155–160. https://doi.org/10.1016/j.colsurfb.2009.03.007
- Coca, J., Gutierrez, G., & Benito, J. (2011). TREATMENT OF OILY WASTEWATER (pp. 1–55). https://doi.org/10.1007/978-90-481-9775-0_1
- COCA, J., GUTIÉRREZ, G., & BENITO, J. (2011). TREATMENT OF OILY WASTEWATER (pp. 1–55). https://doi.org/10.1007/978-90-481-9775-0_1
- Daniel-David, D., le Follotec, A., Pezron, I., Dalmazzone, C., Noïk, C., Barré, L., & Komunjer, L. (2008). Destabilisation of Water-in-Crude Oil Emulsions by Silicone Copolymer Demulsifiers. Oil & Gas Science and Technology - Revue de l’IFP, 63(1), 165–173. https://doi.org/10.2516/ogst:2008002
- Feitosa, F. X., Alves, R. S., & de Sant’Ana, H. B. (2019). Synthesis and application of additives based on cardanol as demulsifier for water-in-oil emulsions. Fuel, 245, 21–28. https://doi.org/10.1016/j.fuel.2019.02.081
- Hirasaki, G. J., Miller, C. A., Raney, O. G., Poindexter, M. K., Nguyen, D. T., & Hera, J. (2011). Separation of Produced Emulsions from Surfactant Enhanced Oil Recovery Processes. Energy & Fuels, 25(2), 555–561. https://doi.org/10.1021/ef101087u
- Holland, P. M., & Rubingh, D. N. (1992). Mixed Surfactant Systems (pp. 2–30). https://doi.org/10.1021/bk-1992-0501.ch001
- Huang, B., Li, X., Zhang, W., Fu, C., Wang, Y., & Fu, S. (2019). Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding. Polymers, 11(3), 395. https://doi.org/10.3390/polym11030395
- Israelachvili, J. (1994). The science and applications of emulsions — an overview. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 91, 1–8. https://doi.org/10.1016/0927-7757(94)02743-9
- Komaiko, J. S., & McClements, D. J. (2016). Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Comprehensive Reviews in Food Science and Food Safety, 15(2), 331–352. https://doi.org/10.1111/1541-4337.12189
- Kronberg, B., Holmberg, K., & Lundman, B. (2014). Mixed Surfactant Systems. In Surface Chemistry of Surfactants and Polymers (pp. 251–269). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781118695968.ch13
- Kumar, N., & Mandal, A. (2018). Surfactant Stabilized Oil-in-Water Nanoemulsion: Stability, Interfacial Tension, and Rheology Study for Enhanced Oil Recovery Application. Energy & Fuels, 32(6), 6452–6466. https://doi.org/10.1021/acs.energyfuels.8b00043
- Li, Z., Kang, W., Bai, B., Wu, H., Gou, C., Yuan, Y., Xu, D., Lu, Y., & Hou, J. (2019). Fabrication and Mechanism Study of the Fast Spontaneous Emulsification of Crude Oil with Anionic/Cationic Surfactants as an Enhanced Oil Recovery (EOR) Method for Low-Permeability Reservoirs. Energy & Fuels, 33(9), 8279–8288. https://doi.org/10.1021/acs.energyfuels.9b01796
- Liden, T., Clark, B. G., Hildenbrand, Z. L., & Schug, K. A. (2017). Unconventional Oil and Gas Production: Waste Management and the Water Cycle (pp. 17–45). https://doi.org/10.1016/bs.apmp.2017.08.012
- Liden, T., Santos, I. C., Hildenbrand, Z. L., & Schug, K. A. (2019). Analytical Methods for the Comprehensive Characterization of Produced Water (pp. 199–217). https://doi.org/10.1016/B978-0-12-815730-5.00009-0
- Liu, J., Huang, X., Lu, L., Li, M., Xu, J., & Deng, H. (2011). Turbiscan Lab® Expert analysis of the biological demulsification of a water-in-oil emulsion by two biodemulsifiers. Journal of Hazardous Materials, 190(1–3), 214–221. https://doi.org/10.1016/j.jhazmat.2011.03.028
- Marquez-Silva, R. L., Key, S., Marino, J., Guzman, C., & Buitriago, S. (1997, February 18). Chemical Dehydration: Correlations between Crude Oil, Associated Water and Demulsifier Characteristics, in Real Systems. All Days. https://doi.org/10.2118/37271-MS
- Mengual, O. (1999). TURBISCAN MA 2000: multiple light scattering measurement for concentrated emulsion and suspension instability analysis. Talanta, 50(2), 445–456. https://doi.org/10.1016/S0039-9140(99)00129-0
- Mengual, O., Meunier, G., Cayre, I., Puech, K., & Snabre, P. (1999). Characterisation of instability of concentrated dispersions by a new optical analyser: the TURBISCAN MA 1000. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 152(1–2), 111–123. https://doi.org/10.1016/S0927-7757(98)00680-3
- Mhatre, S., Simon, S., Sjöblom, J., & Xu, Z. (2018). Demulsifier assisted film thinning and coalescence in crude oil emulsions under DC electric fields. Chemical Engineering Research and Design, 134, 117–129. https://doi.org/10.1016/j.cherd.2018.04.001
- Paweł, B., Zbigniew, J., & Anna, M. (2020). Evaluation of the chemical stability of diesel oil with using Turbiscan Stability Index (TSI). The Archives of Automotive Engineering, 88(2), 5–18.
- Raya, S. A., Mohd Saaid, I., Abbas Ahmed, A., & Abubakar Umar, A. (2020). A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. Journal of Petroleum Exploration and Production Technology, 10(4), 1711–1728. https://doi.org/10.1007/s13202-020-00830-7
- Razi, M., Rahimpour, M. R., Jahanmiri, A., & Azad, F. (2011). Effect of a Different Formulation of Demulsifiers on the Efficiency of Chemical Demulsification of Heavy Crude Oil. Journal of Chemical & Engineering Data, 56(6), 2936–2945. https://doi.org/10.1021/je2001733
- Scanlon, B. R., Weingarten, M. B., Murray, K. E., & Reedy, R. C. (2019). Managing Basin‐Scale Fluid Budgets to Reduce Injection‐Induced Seismicity from the Recent U.S. Shale Oil Revolution. Seismological Research Letters, 90(1), 171–182. https://doi.org/10.1785/0220180223
- Schramm, L. L. (1992). Petroleum Emulsions (pp. 1–49). https://doi.org/10.1021/ba-1992-0231.ch001
- Shu, G., Bu, K., Zhao, B., & Zheng, S. (2021). Evaluation of newly developed reverse demulsifiers and cationic polyacrylamide flocculants for efficient treatment of oily produced water. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 610, 125646. https://doi.org/10.1016/j.colsurfa.2020.125646
- Sjoblom, J. (2001). Encyclopedic Handbook of Emulsion Technology (J. Sjoblom, Ed.). CRC Press. https://doi.org/10.1201/9780367801281
- Tadros, T. F. (2013). Emulsion Formation, Stability, and Rheology. In Emulsion Formation and Stability (pp. 1–75). Wiley. https://doi.org/10.1002/9783527647941.ch1
- Veil, J. A., Puder, M. G., Elcock, D., & Redweik, R. J. , Jr. (2004). A white paper describing produced water from production of crude oil, natural gas, and coal bed methane. https://doi.org/10.2172/821666
- Wang, J., Hu, F.-L., Li, C.-Q., Li, J., & Yang, Y. (2010). Synthesis of dendritic polyether surfactants for demulsification. Separation and Purification Technology, 73(3), 349–354. https://doi.org/10.1016/j.seppur.2010.04.021
- Wu, J., Xu, Y., Dabros, T., & Hamza, H. (2004). Development of a method for measurement of relative solubility of nonionic surfactants. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 232(2–3), 229–237. https://doi.org/10.1016/J.COLSURFA.2003.10.028
- Xu, B., Kang, W., Wang, X., & Meng, L. (2013). Influence of Water Content and Temperature on Stability of W/O Crude Oil Emulsion. Petroleum Science and Technology, 31(10), 1099–1108. https://doi.org/10.1080/10916466.2010.551812
- Yao, L., Selmi, A., & Esmaeili, H. (2021). A review study on new aspects of biodemulsifiers: Production, features and their application in wastewater treatment. Chemosphere, 284, 131364. https://doi.org/10.1016/j.chemosphere.2021.131364
- Yi, M., Huang, J., & Wang, L. (2017). Research on Crude Oil Demulsification Using the Combined Method of Ultrasound and Chemical Demulsifier. Journal of Chemistry, 2017, 1–7. https://doi.org/10.1155/2017/9147926
- Zheng, L., Cao, C., Li, R.-Y., Cao, L.-D., Zhou, Z.-L., Li, M., & Huang, Q.-L. (2018). Preparation and characterization of water-in-oil emulsions of isoprothiolane. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 537, 399–410. https://doi.org/10.1016/j.colsurfa.2017.10.031
- Zhu, Q., Li, J., Liu, H., Saito, M., Tatsumi, E., & Yin, L. (2015). Development of Stable Water-in-Oil Emulsions Using Polyglycerol Polyricinoleate and Whey Protein Isolate and the Impact on the Quality of Bittern-Tofu. Journal of Dispersion Science and Technology, 36(11), 1548–1555. https://doi.org/10.1080/01932691.2014.964360




