Realistic Visualization of Solubility by the Particle Model for Chemistry Education
Published 2019-12-16
Keywords
- Chemistry education,
- Intermolecular Interaction,
- Mixtures,
- Molecular Dynamics,
- Particle Model
- Solubility ...More
How to Cite
Copyright (c) 2019 Antonella Di Vincenzo, Michele Floriano

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
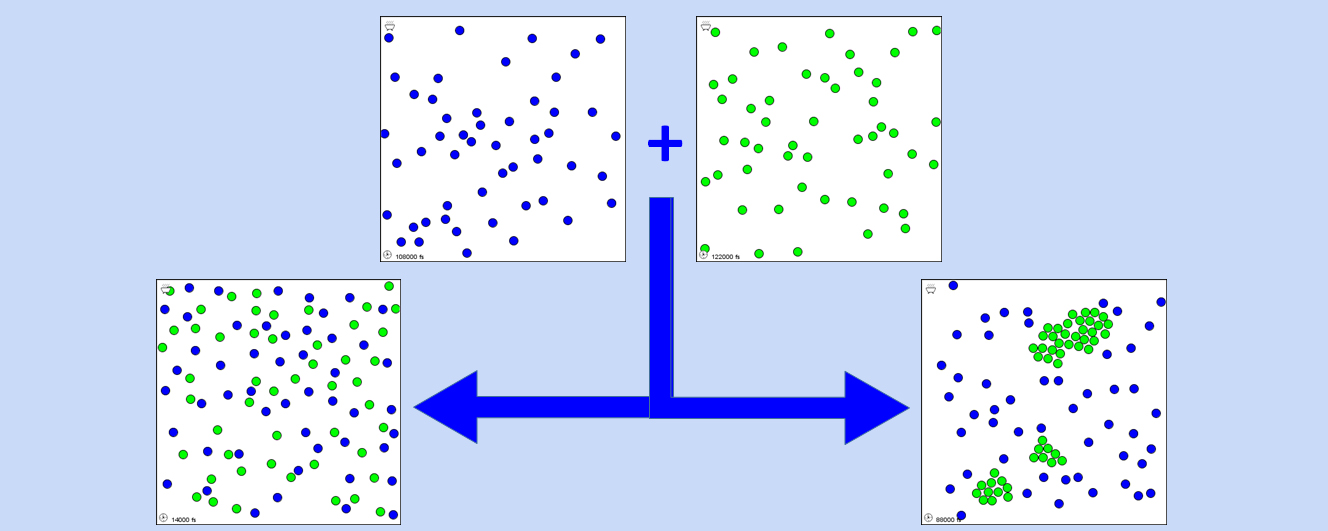
An application for the visualization of the mixing process of two different types of structureless interacting particles is presented. The application allows to demonstrate on a qualitative basis, as well as by quantitatively monitoring the time evolution of the fractions of aggregates of different sizes, that the formation of a homogeneous mixture is the result of favorable solute-solvent interactions as well as by temperature. It is suggested that, along with the use of suitable macroscopic examples, visualizations by the present application are useful in elucidating concepts related to miscibility/solubility. The application is based on a two-dimensional realistic dynamic model where atoms move because of their thermal and interaction potential energies and their trajectories are determined by solving numerically Newton’s laws according to a Molecular Dynamics (MD) scheme. For this purpose, a web-based MD engine was adapted as needed. It is suggested that, when possible, using a realistic simulation rather than simple animations offers several advantages in the visualization of processes of interest in chemistry education. First of all, in a simulation the outcome of the process under study is not set a priori but it is the result of the dynamic evolution of the system; furthermore, specific parameters can be systematically varied and the effects of these changes can be investigated. The application can be used at different levels of detail and in different instruction levels. Qualitative visual observations of the obtained mixtures are suitable at all levels of instruction. Systematic investigations on the effect of changes in temperature and interaction parameters, suitable for senior high school and college courses, are also reported.





