Published 2021-03-22
Keywords
- Zwitterionic polymer resin,
- polyampholytic resins,
- desalinisation,
- ion-exchange resin,
- ammonium bicarbonate
How to Cite
Copyright (c) 2020 Tanita Gettongsong, Mojtaba Taseidifar, Richard M. Pashley, Barry W. Ninham

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
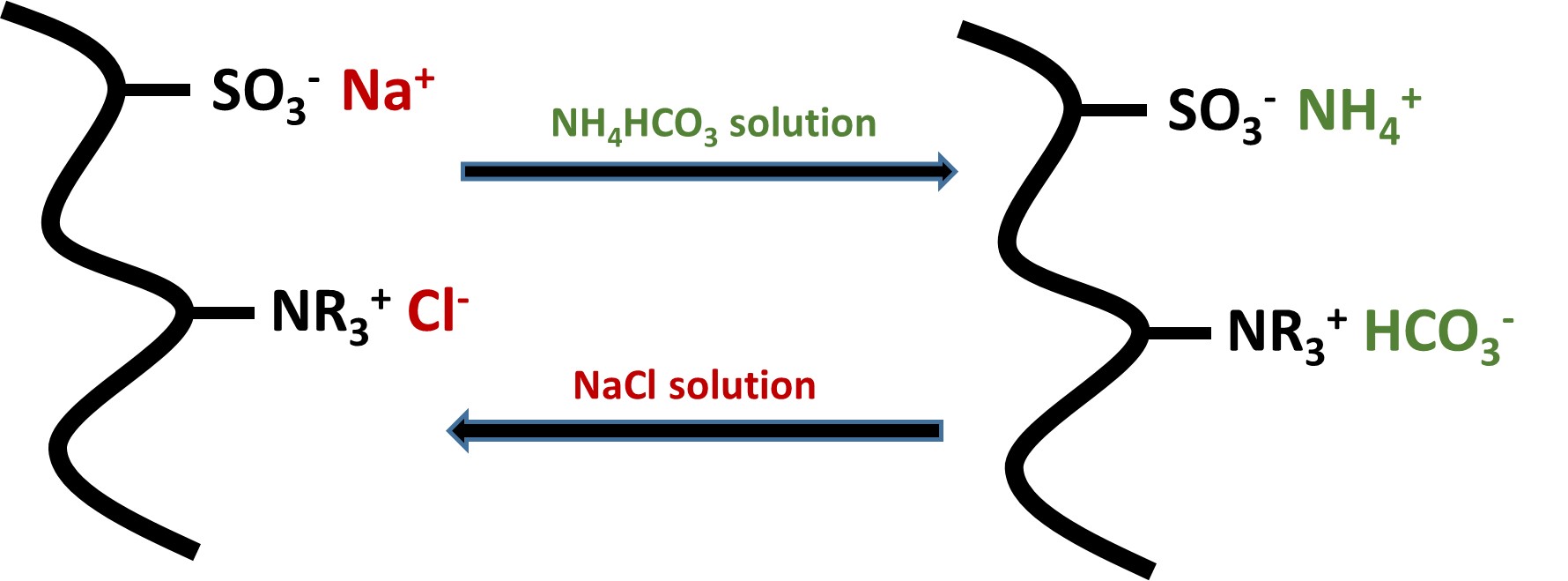
This paper reports the synthesis and properties of new polymer resins containing strong acid and base groups for optimising applications in desalination. Several polyampholytic gels were synthesised with a ratio of 1:1 of strong acid (sulphonate) and strong base (quaternary ammonium) groups and a zwitterionic resin with a 1:1 strong acid and base ratio. The physico-chemical properties of these highly charged resins were studied in electrolyte solutions over a range of pH values, in particular: effects of chemical cross-linking, water and electrolyte swelling; bulk electrical conductivities and surface charging properties in different pH values. The results from absorption of NaCl showed that the resins have considerable potential for more effective desalination than other resin-based techniques.
References
- T.L. Sun, T. Kurokawa, S. Kuroda, A.B. Ihsan, T. Akasaki, K. Sato, M.A. Haque, T. Nakajima, J.P. Gong, Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity, Nat Mater, 2013, 12(10), 932-7.
- L. Su, S. Khan, J. Fan, Y.-N. Lin, H. Wang, T.P. Gustafson, F. Zhang, K.L. Wooley, Functional sugar-based polymers and nanostructures comprised of degradable poly(d-glucose carbonate)s, Poly. Chem., 2017, 8(10), 1699-1707.
- D. Tatini, F. Sarri, P. Maltoni, M. Ambrosi, E. Carretti, B.W. Ninham, P. Lo Nostro, Specific ion effects in polysaccharide dispersions, Carbohydr. Polym., 2017, 173, 344-352.
- C.A. Finch, Polymers in aqueous media: Performance through association. Advances in Chemistry Series No. 223 Edited by J. E. Glass, ACS, Washington, Polym. Int., 1991, 25(1), 61-62.
- M.A. Hubbe, O.J. Rojas, D.S. Argyropoulos, Y. Wang, J. Song, N. Suli?, T. Sezaki, Charge and the dry-strength performance of polyampholytes: Part 2. Colloidal effects, Colloids Surf., A:Physiochem. Eng. Aspects, 2007, 301(1), 23-32.
- A.B. N. Alepee, M. Daneshian, B. De Wever, E. Fritsche, A. Goldberg, J. Hansmann, T. Hartung, J. Haycock, H.T. Hogberg, t4 workshop report: State-of-the-art of 3D cultures (organs-on-a-chip) in safety testing and pathophysiology, Altex, 2014, 31(4), 441-477.
- J. H. Chen, C. C. Tsai, Y.Z. Kehr, L. Horng, K. Chang, L. Kuo, An Experimental Study of Drag Reduction in a Pipe with Superhydrophobic Coating at Moderate Reynolds Numbers, in ICEM 14 – 14th International Conference on Experimental Mechanics. EPJ Web of Conferences Poitiers, France, 2010.
- C. Zhang, C. Lai, G. Zeng, D. Huang, C. Yang, Y. Wang, Y. Zhou, M. Cheng, Efficacy of carbonaceous nanocomposites for sorbing ionizable antibiotic sulfamethazine from aqueous solution, Water Res., 2016, 95, 103-112.
- S.E. Kudaibergenov, A. Ciferri, Natural and Synthetic Polyampholytes, 2, Macromol. Rapid Commun., 2007, 28(20), 1969-1986.
- N.P.G.N. Chandrasekara, R.M. Pashley, A model for ion-exchange behaviour of polyampholytic resins: Using polystyrene polyampholytic latex, Colloids Surf., A:Physiochem. Eng. Aspects, 2017, 516, 39-47.
- D.S. Eldridge, R.J. Crawford, I.H. Harding, The role of metal ion-ligand interactions during divalent metal ion adsorption, J. Colloid Interface Sci., 2015, 454, 20-26.
- A. Homola, R.O. James, Preparation and characterization of amphoteric polystyrene latices, J. Colloid Interface Sci., 1977, 59(1), 123-134.
- E. Ruckenstein, M. Manciu, Stability of dispersions, in Nanodispersions: Interactions, Stability, and Dynamics, Springer New York: New York, NY, 2010, 201-324.
- S. Salgin, U. Salgin, S. Bahadir, Zeta potentials and isoelectric points of biomolecules: The effects of ion types and ionic strengths, Int. J. Electrochem. Sci., 2012, 7(12), 12404-12414.
- S. Perez-Amodio, P. Holownia, C.L. Davey, C.P. Price, Effects of the ionic environment, charge, and particle surface chemistry for enhancing a latex homogeneous immunoassay of C-reactive protein, Anal. Chem., 2001, 73(14), 3417-3425.
- I.H. Harding, T.W. Healy, Adsorption of aqueous cadmium(II) on amphoteric latex colloids: I. General kinetics and thermodynamics, J. Colloid Interface Sci., 1985, 107(2), 362-370.
- I.H. Harding, T.W. Healy, Adsorption of aqueous cadmium(II) on amphoteric latex colloids: II. Isoelectric point effects, J. Colloid Interface Sci., 1985, 107(2), 371-381.
- M. Chanda, S.A. Pillay, A. Sarkar, J.M. Modak, A thermally regenerable composite sorbent of crosslinked poly(acrylic acid) and ethoxylated polyethyleneimine for water desalination by Sirotherm process, J. Appl. Polym. Sci., 2009, 111(6), 2741-2750.
- N.P.G.N. Chandrasekara, R.M. Pashley, Study of a new process for the efficient regeneration of ion exchange resins, Desalination, 2015, 357, 131-139.
- B.A. Bolto, R. McNeill, A.S. MacPherson, R. Siudak, D.E. Weiss, D. Willis, An Ion-Exchange Process with Thermal Regeneration. VI. Factors Influencing the Titration Curve Shape of Weak Electrolyte Resins, Australian J. Chem., 1968, 21(11), 2703-2710.
- D.E. Weiss, B.A. Bolto, R. McNeill, A.S. MacPherson, R. Siudak, E.A. Swinton, D. Willis, An Ion-Exchange Process with Thermal Regeneration. IV. Equilibria In A Mixed Bed of Weak-Electrolyte Resins, Australian J. Chem., 1966, 19(5), 765-789.
- D. Weiss, B. Bolto, R. McNeill, A. MacPherson, R. Siudak, E. Swinton, D. Willis, An ion-exchange process with thermal regeneration. II. Properties of weakly basic resins, Australian J. Chem., 1966, 19(4), 561-587.
- D.E. Weiss, B.A. Bolto, R. McNeill, A.S. MacPherson, R. Siudak, E.A. Swinton, D. Willis, An ION-Exchange Process with Thermal Regeneration. III. Properties of Weakly Acidic ION-Exchange Resins, Australian J. Chem., 1966, 19(4), 589-608.
- V.K. Koul, A.K. Gupta, Uptake of sodium chloride by mixture of weakly acidic and weakly basic ion exchange resins: equilibrium and kinetic studies, Chem. Eng. Sci., 2004, 59(7), 1423-1435.
- J.T. Duniec, J.N. Israelachvili, B.W. Ninham, R.M. Pashley, S.W. Thorne, An ion-exchange model for thylakoid stacking in chloroplasts, FEBS Letters, 1981, 129(2), 193-196.
- R.M. Pashley, DLVO and hydration forces between mica surfaces in Li+, Na+, K+, and Cs+ electrolyte solutions: A correlation of double-layer and hydration forces with surface cation exchange properties, J. Colloid Interface Sci., 1981, 83(2), 531-546.
- F. Makavipour, R.M. Pashley, A study of ion adsorption onto surface functionalized silica particles, Chem. Eng. J., 2015, 262, 119-124.
- S. Durmaz, O. Okay, Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: synthesis and characterization, Polym., 2000, 41(10), 3693-3704.
- L. Zhang, A. Eisenberg, Formation of crew-cut aggregates of various morphologies from amphiphilic block copolymers in solution, Polym. Adv. Technol.s, 1998, 9(10?11), 677-699.
- A. El-Hag Ali, H.A. Shawky, H.A. Abd El Rehim, E.A. Hegazy, Synthesis and characterization of PVP/AAc copolymer hydrogel and its applications in the removal of heavy metals from aqueous solution, Europ. Polym. J., 2003, 39(12), 2337-2344.
- A.M. Atta, H.S. Ismail, A.M. Elsaaed, Application of anionic acrylamide-based hydrogels in the removal of heavy metals from waste water, J. Appl. Polym. Sci., 2012, 123(4), 2500-2510.
- N. Yan, D.R. Paul, B.D. Freeman, Water and ion sorption in a series of cross-linked AMPS/PEGDA hydrogel membranes, Polym., 2018, 146, 196-208.
- R.M. Pashley, M. Taseidifar , T. Gettongsong, Resin for desalination and process of regeneration, PCT, Google Patents, 2019.
- N. Tarannum, M. Singh, Synthesis and characterization of zwitterionic organogels based on Schiff base chemistry, J. Appl. Polym. Sci., 2010, 118(5), 2821-2832.
- B.W. Ninham, R.M. Pashley, P. Lo Nostro, Surface forces: Changing concepts and complexity with dissolved gas, bubbles, salt and heat, Curr. Opin. Colloid Interface Sci., 2017, 27, 25-32.
- F. Cugia, M. Monduzzi, B.W. Ninham, A. Salis, Interplay of ion specificity, pH and buffers: insights from electrophoretic mobility and pH measurements of lysozyme solutions, RSC Advances, 2013, 3(17), 5882-5888.
- A. Salis, L. Cappai, C. Carucci, D.F. Parsons, M. Monduzzi, Specific Buffer Effects on the Intermolecular Interactions among Protein Molecules at Physiological pH, J. Phy. Chem. Lett., 2020, 11(16), 6805-6811.




