Published 2021-03-22
Keywords
- Themal decomposition,
- bubble column evaporator,
- zwitterionic polymer resin,
- desalinisation,
- ion-exchange resin
- ammonium bicarbonate,
- hollow fibre membrane ...More
How to Cite
Copyright (c) 2020 Tanita Gettongsong, Mojtaba Taseidifar, Richard M. Pashley

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
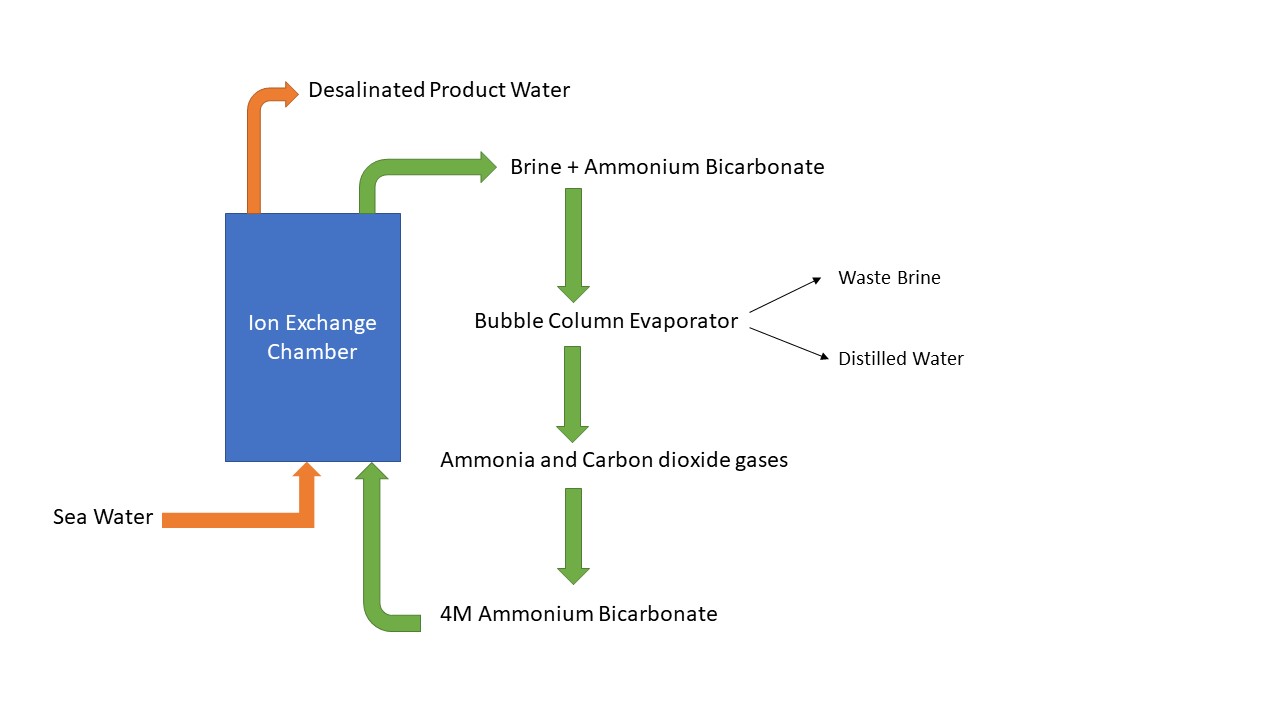
The report is concerned with the design and synthesis of a mixed bead resin for high salt level desalination. The resin allows for the simultaneous exchange of both anions and cations, within the same polymer. This improves the efficiency of desalination at seawater levels. A novel process for sustainable and low energy desalination for brackish water has already been achieved via ion exchange resins as explained below. The advance in resin technology improves a novel membrane process with closed–cycle regeneration of the resin. It is a superior alternative to reverse osmosis.
References
- I. C. Karagiannis, P. G. Soldatos, Water desalination cost literature: review and assessment, Desalination, 2008, 223, 448-456.
- N. P. G. N. Chandrasekara, R. M. Pashley, Regeneration of strong acid/strong base mixed-bed resins using ammonium bicarbonate (AB) for a sustainable desalination process, Desalination, 2017, 409, 1-6.
- N. P. G. N. Chandrasekara, R. M. Pashley, Study of a new process for the efficient regeneration of ion exchange resins, Desalination, 2015, 357, 131-139.
- N. Tarannum, M. Singh, Synthesis and characterization of zwitterionic organogels based on Schiff base chemistry, J. Appl. Polym. Sci., 2010, 118, 2821-2832.
- N. P. G. N. Chandrasekara, R. M. Pashley, Enhanced ion exchange capacity of polyampholytic resins, Sep. Purif. Technol., 2016, 158, 16-23.
- G. W. Gokel, Dean's handbook of organic chemistry, McGraw-Hill, New York, 2004.
- M. Shahid, X. Xue, C. Fan, B. W. Ninham, R. M. Pashley, Study of a novel method for the thermolysis of solutes in aqueous solution using a low temperature bubble column evaporator, J. Phys. Chem. B, 2015, 119, 8072–8079.
- V. S. J. Craig, B. W. Ninham, R. M. Pashley, The effect of electrolytes on bubble coalescence in water, J. Phys. Chem., 1993, 97, 10192-10197.




