A Study of the Bubble Column Evaporator Method for Improved Ammonium Bicarbonate Decomposition in Aqueous Solutions: Desalination and Other Techniques
Published 2021-03-22
Keywords
- Non-boiling decomposition,
- bubble coalescence,
- transient collisions,
- ammonium bicarbonate
How to Cite
Copyright (c) 2020 Muhammad Shahid, Mojtaba Taseidifar , Richard M. Pashley

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
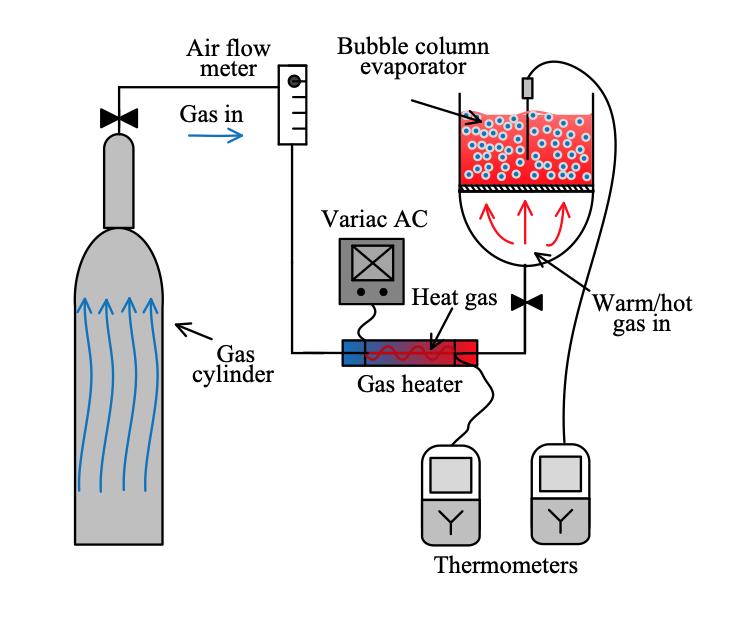
A bubble column was used to study the improved thermal decomposition of NH4HCO3 in aqueous solution using a continuous flow of hot gas bubbles of optimum sizes (1-3 mm) produced via controlled bubble coalescence to maintain bubble size. The rapid transfer of heat from small, hot (dry) gas bubbles to the surrounding water, i.e. into a transient hot surface layer, was used as an effective and energy efficient method of decomposing ammonium bicarbonate in aqueous solution. It is shown that the continuous flow of (dry) hot gases, even at 275°C, only heat the aqueous solution in the bubble column to about 57°C, at which it was also established that NH4HCO3 has a negligible decomposition rate even with long-term exposure to this solution temperature. Hence, the effects observed appeared to be caused entirely by the effective collisions between the hot gas bubbles and the solute. It was also established that the use of high gas inlet temperatures can reduce the thermal energy requirement to only about 50% (i.e. about 575 kJ/L) of that reported in previous studies and less than 25% of solution boiling.
References
- J. R. McCutcheon, R. L. McGinnis, and M. Elimelech, A novel ammonia—carbon dioxide forward (direct) osmosis desalination process, Desalination, 2005, 174(1), 1-11.
- N. P. G. N. Chandrasekara and R. M. Pashley, Study of a new process for the efficient regeneration of ion exchange resins, Desalination, 2015, 357, 131-139.
- M. Shahid, X. Xue, C. Fan, B.W. Ninham, and R.M. Pashley, Study of a novel method for the thermolysis of solutes in aqueous solution using a low temperature bubble column evaporator, J. Phys. Chem. B, 2015, 119 (25), 8072–8079.
- G. Fulks, G. B. Fisher, K. Rahmoeller, M. C. Wu, E. D’Herde, J. Tan, A review of solid materials as alternative ammonia sources for lean NOx reduction with SCR, 2009, SAE Technical Paper.
- G. W. Gokel, Dean's handbook of organic chemistry. McGraw-Hill New York, 2004, 71375937.
- P. Zehner and M. Kraume, Bubble columns, Ullmann's Encyclopedia of Industrial Chemistry, 2000.
- C. Fan, M. Shahid, R.M. Pashley, Studies on bubble column evaporation in various salt solutions, J. Sol. Chem., 2014, 43(8), 1297-1312.
- M. Francis, R. Pashley, Application of a Bubble Column for Evaporative Cooling and a Simple Procedure for Determining the Latent Heat of Vaporization of Aqueous Salt Solutions, J. Phys. Chem. B, 2009, 113(27), 9311-9315.
- V. S. J. Craig, B. W. Ninham, R. M. Pashley, The effect of electrolytes on bubble coalescence in water, J. Phys. Chem. , 1993, 97(39), 10192-10197.
- M. Shahid, C. Fan, R. M. Pashley, Insight into the bubble column evaporator and its applications, Int. Rev. Phys. Chem. , 2016, 35(1), 143-185.
- C. Fan and R. M. Pashley, Precise Method for Determining the Enthalpy of Vaporisation of Concentrated Salt Solutions Using a Bubble Column Evaporator, J. Sol. Chem., 2015, 44(1), 131-145.
- M. J. Francis, R. M. Pashley, Thermal desalination using a non-boiling bubble column, Desalin. Water Treat., 2009, 12(1-3), 155-161.
- M. Shahid, R. M. Pashley, A study of the bubble column evaporator method for thermal desalination, Desalination, 2014, 351, 236-242.
- M. Taseidifar, M. Shahid, R. M. Pashley, A study of the bubble column evaporator method for improved thermal desalination, Desalination, 2018, 432, 97-103.
- X. Xue and R.M. Pashley, A study of low temperature inactivation of fecal coliforms in electrolyte solutions using hot air bubbles, Desalin. Water Treat., 2015, 1–11.
- M. Shahid, A study of the bubble column evaporator method for improved sterilization, J. Water Process. Eng., 2015, 8, 1–6.
- A. G. Sanchis, M. Shahid, and R. M. Pashley, Improved virus inactivation using a hot bubble column evaporator (HBCE), Colloids and Surfaces B: Biointerfaces, 2018, 165, 293-302.
- A. G. Sanchis, R. M. Pashley, B. W. Ninham, Virus and bacteria inactivation by CO2 bubbles in solution, NPJ Clean Water, 2019, 2(1), 5.
- M. Shahid, R. M. Pashley, M. Rahman, Use of a high density, low temperature, bubble column for thermally efficient water sterilisation, Desalin. Water Treat., 2014, 52, 4444–4452.
- C. Fan, R. M. Pashley, The controlled growth of calcium sulfate dihydrate (gypsum) in aqueous solution using the inhibition effect of a bubble column evaporator, Chem. Eng. Sci., 2016, 142, 23-31.
- M. Taseidifar, F. Makavipour, R. M. Pashley, A. F. M. M. Rahman, Removal of heavy metal ions from water using ion flotation, Environ. Technol. Innov., 2017, 8, 182-190.
- P. N. Govindan, G. P. Thiel, R. K. McGovern, J. H. Lienhard, M. H. Elsharqawy, Bubble-Column Vapor Mixture Condenser. Google Patents.
- G. P. Narayan, J. H. Lienhard, Thermal Design of Humidification–Dehumidification Systems for Affordable Small-Scale Desalination, IDA J. Desalin. Water Reuse, 2012, 4(3), 24-34.
- M. Schmack, H. Goen, and A. Martin, A Bubble Column Evaporator with Basic Flat-plate Condenser for Brackish and Seawater Desalination, Environ. Technol., 2015 37(1), 74–85.
- C. P. Ribeiro, P. L. C. Lage, Gas?Liquid Direct?Contact Evaporation: A Review, Chem. Eng. Technol., 2005, 28(10), 1081-1107.




