Capillary Electrophoresis (CE) and its Basic Principles in Historical Retrospect. Part 3. 1840s –1900ca. The First CE of Ions in 1861. Transference Numbers, Migration Velocity, Conductivity, Mobility.
Published 2022-03-07
Keywords
- First Capillary Electrophoresis,
- Ions,
- Strong Electrolytes,
- Hittorf,
- Clausius
- Kohlrausch ...More
How to Cite
Copyright (c) 2022 Ernst Kenndler

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
Since electrophoresis is a physical phenomenon – it is the movement of dispersed charged particles relative to a liquid under the influence of a spatially uniform electric field - its history is not limited to its use as a separation method. The history of capillary electrophoresis in particular, i.e. electrophoresis in capillary-sized open tubes, therefore does not begin in the 1960s, as is commonly assumed, but already a century earlier, if one refers to its principles
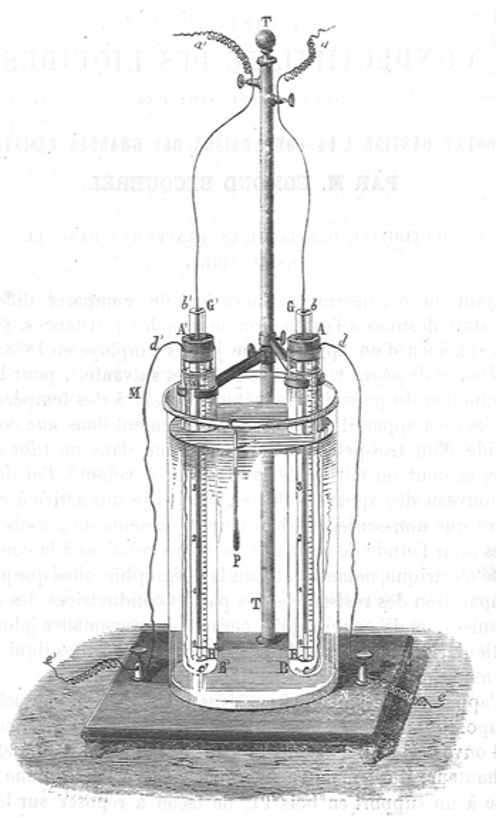
Capillary electrophoresis of ions was first performed by the French physicist Edmond Becquerel in 1861, about the same year as that of colloidal particles. Becquerel owns therefore the priority. It was subsequently performed on three other occasions in the Long Nineteenth Century, by Wilhelm Beetz in 1865, by Wilhelm Ostwald and Walther Nernst in 1889, and by Friedrich Kohlrausch and Adolf Heydweiller in 1895. All of these experiments were carried out in the context of research on conductivity and ion migration.
Based on the theories of Grotthuß, Davy, and Faraday, it was believed until the 1840s that both the anions and the cations of a dissolved strong electrolyte - to which this review refers - travel at the same speed in an electric field.), migrate at the same velocity or speed in an electric field, but experimental observations in the mid-1840s cast doubt on this view. Wilhelm Hittorf was the first to show that these ions could migrate at different speeds, still consistent with Faraday´s laws. He was able to prove his hypothesis with experimental data and determined the migration velocities of the two types of ions in an electrolyte relative to the sum of their velocities, which he termed "Überführungszahlen" (transference or transport numbers). However, they did not initially yield the absolute velocities of the ions. This was achieved later by F. Kohlrausch, who devoted four decades of his research life, namely from the end of 1860 to about 1910, to the study of the conductivity of electrolyte solutions and the migration of ions. He discovered in 1879, that ions move independently from each other in solution (1st Kohlrausch law).
It is remarkable that until the late 1880s it was generally believed that free ions do not exist in solutions in the absence of an external electrical force, but that ions were always tightly bound to their counterions. This belief dated back to Grotthuß in 1805. Although Rudolf Clausius hypothesized in 1857 that free ions are actually present in solutions as result of their thermal motion, this did not find further resonance. It is also remarkable that during this whole period under consideration no attempt was ever made to separate ions with the same charge, although their different migration properties were already known.
Continuing his research, Kohlrausch found empirically in 1900 that at extremely low concentrations the molar conductivity of ions, i.e. the conductivity related to their concentration, is a function of the square root of their concentration and approaches a certain limit at infinite dilution (2nd Kohlrausch law). As a precursor to this law, he derived in 1885 for larger concentration ranges the little-known relationship of molar conductivity as a function of the cubic root of concentration. He calculated the migration velocities of ions from their conductivities and characterized the migration behavior by their mobility, which is a central property in electrophoresis.
Kohlrausch was certainly a formative investigator of the electrophoretic properties of ions, but his work focused mainly on strong electrolytes. This review covers the research results in this field in the period from 1840 to about 1910, but also reports on the historical background at this time and the personal background of some researchers who, despite important contributions, have been unjustly forgotten, as well as on researchers who were active outside the scientific community. Mention is made, for example, of Gustav Theodor Fechner, who was the first to prove the fact, indispensable for electrophoresis, that Ohm´s law also applies to electrolyte solutions. However, in contrast to the generally applied results of his investigations, he himself was rather ignored by later researchers.
The conductivities and electrophoretic properties of weak electrolytes, which were known to Kohlrausch and his contemporaries but hardly explicable to them, at least until 1884, are not discussed in detail in this review. In that year, Svante Arrhenius published his groundbreaking dissociation theory. This theory and the resulting consequences for the whole subject of electrolyte solutions require, however, a separate historical retrospect.
References
- E. Kenndler, M. Minárik, Substantia 2021, 5 (1), 119-133.
- J. Lyklema, Fundamentals of Interface and Colloid Science. Solid-Liquid Interfaces, Vol. 2, Academic Press, London, San Diego, 1995.
- J. H. Lyklema, Substantia 2017, 1 (2), 75-93.
- E. Kenndler, Substantia 2021, 5 (2), 95-118.
- J. C. Maxwell, Phil. Trans. 1865, 155, 459–512.
- P. M. Roget, Electricity, Galvanism, Magnetism, and Electro-Magnetism, Robert Baldwin, London, 1831.
- P. M. Roget, Treatises on Electricity, Galvanism, Magnetism, and Electro-magnetism, Baldwin and Cradock, London, 1832.
- M. Faraday, Phil. Trans. 1833, 123, 675-710.
- M. Faraday, Ann. Phys. Chem. (Pogg.) 1834, 32, 401-453.
- M. Faraday, Phil. Trans. 1834, 124, 425-470.
- M. Faraday, Phil. Trans. 1838, 128, 125-168.
- W. Watson, Phil. Trans. 1748, 45, 49-92.
- W. Watson, Phil. Trans. 1748, 45, 93-121.
- C. Wheatstone, Phil. Trans. 1834, 124, 583–591.
- J. F. Daniell, W. A. Miller, Phil. Trans. 1844, 134, 1-20.
- J. F. Daniell, W. A. Miller, Ann. Chem. Phys. (Pogg.) 1845, 64, 18-48.
- C. S. M. Pouillet, Élémens de physique expérimentale et de météorologie, Vol. 1, 1 ed., Béchet Jeune, Paris, 1827.
- C. S. M. Pouillet, Comp. rend. 1838, 12, 24-65.
- C. S. M. Pouillet, Comp. rend. 1845, XX, 1544-1549.
- C. S. M. Pouillet, Ann. Phys. Chem. (Pogg.) 1845, 65, 474-476.
- A. Smee, Phil. Mag. 1844, 25 (3. ser.), 434-442.
- A. Smee, Ann. Phys. Chem. (Pogg.) 1845, 65, 470-476.
- W. F. Stevenson, Most important Errors in Chemistry, Electricity, and Magnetism, pointed out and refuted: and the Phenomena of Electricity, and the Polarity of the magnetic Needle accounted for and explained by a Fellow of the Royal Society, 1. ed., James Ridgway, London, 1846.
- W. R. Grove, Phil. Mag. 1838, XIII, 430-431.
- W. R. Grove, Phil. Mag. 1839, XIV, 127-130.
- W. Grove, Comp. rend. 1839, VIII, 567-570.
- C. F. Schönbein, Ann. Phys. Chem. (Pogg.) 1840, 125, 511-514.
- C. F. Schönbein, Ann. Phys. Chem. (Pogg.) 1840, 125, 589-590.
- W. F. Stevenson, The Composition of Hydrogen and the Non-Decomposition of Water incontrovertibly established, in Answer to the Award of a Medal by the Royal Society whereby the Contrary Doctrines are absolutely affirmed, also the Absurdity of the existing Systems of Electricity and Magnetism demonstrated and the True One Given, 2. ed., James Ridgway, London, 1849.
- J. Priestley, The Doctrine of Phlogiston Established and that of the Composition of Water refuted, 2. ed., Andrew Kennedy, Northumberland, 1803.
- M. Faraday, Phil. Trans. 1846, 136, 1-20.
- J. C. Maxwell, Proc. Roy. Soc. Edinburgh 1846, 2, 89-93.
- R. Clausius, Ann. Phys. Chem. (Pogg.) 1850, 79, 368-397.
- J. P. Joule, Phil. Trans. 1850, 140, 61-77.
- C. Darwin, On the Origin of Species by means of Natural Selection, or the Preservation of favoured Races in the Struggle for Life, 1. ed., John Murray, London, 1859.
- W. Hittorf, Ann. Phys. Chem. (Pogg.) 1853, 89, 177-211.
- W. Hittorf, in Harper´s Scientific Memoirs. VII. The Fundamental Laws of Electrolyte Conduction. Memoirs by Faraday, Hittorf and F. Kohlrausch (Ed.: J. S. Ames, Goodwin, H. M.), Harper & Brothers, New York and London, 1899, pp. 49-80.
- J. J. Berzelius, J. Phys. Chim. 1811, 73, 253-286.
- G. Wiedemann, Ann. Phys. Chem. (Pogg.) 1858, 104, 162-170.
- P. Walden, Z. phys. Chem. 1906, 55, 207-249.
- W. Hittorf, Ann. Phys. Chem. (Pogg.) 1856, 98, 1-33.
- W. Hittorf, Ann. Phys. Chem. (Pogg.) 1859, 106, 337-411.
- G. Magnus, Ann. Phys. Chem. (Pogg.) 1857, 102, 1-54.
- G. Magnus, Ann. Phys. Chem. (Pogg.) 1858, 104, 553-580.
- W. Hittorf, Ann. Phys. Chem. (Pogg.) 1859, 106, 513-586.
- R. Clausius, Ann. Phys. Chem. (Pogg.) 1857, 101, 338-360.
- G. Wiedemann, Ann. Phys. Chem. (Pogg.) 1856, 99, 177-233.
- A. Weiske, Ann. Phys. Chem. (Pogg.) 1858, 103, 466-486.
- G. Milazzo, Elektrochemie-Theoretische Grundlagen und Anwendungen, Springer, Wien, 1952.
- F. Kohlrausch, Göttinger Nachr. 1869, 1, 14-16.
- G. S. Ohm, J. Chem. Phys. (Schw.) 1825, 44, 245–247.
- G. S. Ohm, J. Chem. Phys. (Schw.) 1825, 44, 110–118.
- G. S. Ohm, Ann. Phys. Chem. (Pogg.) 1826, 6, 459–469.
- G. S. Ohm, Ann. Phys. Chem. (Pogg.) 1826, 7, 45–54.
- G. S. Ohm, Ann. Phys. Chem. (Pogg.) 1826, 7, 117–118.
- G. S. Ohm, Die galvanische Kette, mathematisch bearbeitet, Riemann, T.H., Berlin, 1827.
- C. S. M. Pouillet, Comp. rend. 1837, 4, 267–279.
- C. S. M. Pouillet, Ann. Phys. Chem. (Pogg.) 1837, 42, 281–296.
- G. S. Ohm, Journ. Phys. Chem. (Schweigg.) 1826, 46, 137-166.
- C. S. M. Pouillet, Ann. Phys. Chem. (Pogg.) 1829, 15, 91-98.
- E. Péclet, Comp. rend. 1845, 20, 54–60.
- F. C. Henrici, Ann. Phys. Chem. (Pogg.) 1841, 53, 277-294.
- F. C. Henrici, Ann. Phys. Chem. (Pogg.) 1841, 54, 412-416.
- G. T. Fechner, https://www.sil.si.edu/DigitalCollections/hst/scientific-identity/fullsize/SIL14-F002-01a.jpg.
- G. T. Fechner, D. Mises, Beweis, daß der Mond aus Jodine bestehe, 1. ed., Germanien (Penig), 1821.
- G. T. Fechner, D. Mises, Vergleichende Anatomie der Engel, 1. ed., Industrie-Comptoir, Leipzig, 1825.
- J.-B. Biot, Précis élémentaire de physique expérimentale, Vol. 1, 2. ed., Deterville Paris, 1821.
- J.-B. Biot, Précis élémentaire de physique expérimentale, Vol. 2, 2. ed., Deterville, Paris, 1821.
- G. T. Fechner, Massbestimmungen über die galvanische Kette, F. A. Brockhaus, Leipzig 1831.
- G. T. Fechner, D. Mises, Gedichte, 1. ed., Breitkopf und Härtel, Leipzig, 1841.
- G. T. Fechner, Nanna, oder über das Seelenleben der Pflanzen, Leopold Voß, Leipzig, 1848.
- G. T. Fechner, Zend-Avesta oder über die Dinge des Himmels und des Jenseits. Vom Standpunkt der Naturbetrachtung. Erster Theil. Ueber die Dinge des Himmels., Vol. 1, Leopold Voß, Leipzig, 1851.
- G. T. Fechner, Zend-Avesta oder über die Dinge des Himmels und des Jenseits. Vom Standpunkt der Naturbetrachtung. Zweiter Theil. Ueber die Dinge des Himmels., Vol. 2, Leopold Voß, Leipzig, 1851.
- G. T. Fechner, Zend-Avesta oder über die Dinge des Himmels und des Jenseits. Vom Standpunkt der Naturbetrachtung. Dritter Theil. Ueber die Dinge des Jenseits., Vol. 3, Leopold Voß, Leipzig, 1851.
- G. T. Fechner, Elemente der Psychophysik, Erster Teil (Elements of Psychophysics, 1st part), Vol. 1, Breitkopf und Härtel, Leipzig, 1860.
- G. T. Fechner, Elemente der Psychophysik, Zweiter Teil (Elements of Psychophysics, 2nd part), Vol. 2, Breitkopf und Härtel, Leipzig, 1860.
- G. T. Fechner, In Sachen der Psychophysik, 1. ed., Breitkopf und Härtel, Leipzig, 1877.
- G. T. Fechner, Revision der Hauptpuncte der Psychophysik, Breitkopf und Härtel, Leipzig, 1882.
- G. T. Fechner, Die Tagesansicht gegenüber der Nachtansicht, Breitkopf und Härtel, Leipzig, 1879.
- G. T. Fechner, Ann. Phys. Chem. (Pogg.) 1874, Jubelband, 66–81.
- J. P. Joule, Phil. Mag. 1841, 19, 260-277.
- M. E. Lenz, Ann. Phys. Chem. (Pogg.) 1844, 61, 18-49.
- E. Becquerel, Ann. Chim. Phys. 1843, 9, 21-70.
- R. Clausius, Ann. Phys. Chem. (Pogg.) 1852, 87, 415-426.
- R. Clausius, in Scientific Memoirs (Eds.: J. Tyndall, W. Francis), Taylor and Francis, London, 1853, pp. 200-209.
- R. Clausius, Ann. Chim. Phys. 1854, XLII (3. série), 122-125.
- R. Clausius, Phil. Mag. 1858, 15, 94-109.
- R. Clausius, Ann. Chim. Phys. 1858, 53, 252-256.
- M. Faraday, Phil. Trans. 1834, 124, 77-122.
- R. Clausius, Ann. Phys. Chem. (Pogg.) 1857, 100, 353-380.
- R. Clausius, Phil. Mag. (ser. 4) 1857, 14, 108-127.
- F. Exner, Ann. Phys. Chem. (N. F., Wied.) 1879, 6, 330-384.
- F. Kohlrausch, Göttingen (Göttingen), 1863.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1863, 119, 337-368.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1865, 125, 626-629.
- F. Kohlrausch, Z. ration. Med. 1866, 28, 190-204.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1866, 128, 1-20.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1866, 128, 399-410.
- F. Kohlrausch, Göttinger Nachr. 1868, 1868, 159-163.
- F. Kohlrausch, Göttinger Nachr. 1869, 1869, 35-42.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1869, 138, 173.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1869, 138, 1-10.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1868, 135, 120-125.
- W. A. Nippoldt, Göttingen (Göttingen), 1869.
- F. Kohlrausch, Göttinger Nachr. 1868, 415-420.
- F. Kohlrausch, W. A. Nippoldt, Ann. Phys. Chem. (Pogg.) 1869, 138, 280-298.
- F. Kohlrausch, W. A. Nippoldt, Ann. Phys. Chem. (Pogg.) 1869, 138, 370-390.
- G. Wiedemann, Die Lehre vom Galvanismus und Elektromagnetismus nebst ihren technischen Anwendungen. Erster Band. Galvanismus, Vol. 1, 1. ed., Friedrich Vieweg und Sohn, Braunschweig, 1861.
- F. Kohlrausch, Z. Elektrochem. 1908, 14, 384-386.
- H. Buff, Ann. Chem. Pharm. 1855, 96, 257-286.
- F. Kohlrausch, O. Grotrian, Ann. Phys. Chem. (Pogg.) 1875, 154, 1-14.
- F. Kohlrausch, O. Grotrian, Ann. Phys. Chem. (Pogg.) 1875, 154, 215-239.
- F. Kohlrausch, Ann. Phys. Chem. (Pogg.) 1876, 159, 233-275.
- F. Kohlrausch, Göttinger Nachr. 1876, 213-224.
- W. G. Hankel, Ann. Phys. Chem. (Pogg.) 1846, 69, 255-264.
- W. Beetz, Ann. Phys. Chem. (Pogg.) 1862, 117, 1-27.
- G. H. L. Hagen, Ann. Phys. Chem. (Pogg.) 1839, 46, 423-442.
- J. L. M. Poiseuille, Comp. rend. 1840, 11, 961-967.
- J. L. M. Poiseuille, Comp. rend. 1840, 11, 1041-1148.
- J. L. M. Poiseuille, Comp. rend. 1841, 12, 112-115.
- G. Wiedemann, Die Lehre vom Galvanismus und Elektromagnetismus. Erster Band. Galvanismus, Vol. 1, 2. ed., Friedrich Vieweg und Sohn, Braunschweig, 1874.
- H. Euler, Ann. Phys. Chem. (N. F., Wied.) 1897, 63, 273-277.
- G. Quincke, Ann. Phys. Chem. (Pogg.) 1871, 144, 1-33.
- O. Grotrian, Ann. Phys. Chem. (Pogg.) 1876, 157, 130-146.
- O. Grotrian, Ann. Phys. Chem. (Pogg.) 1877, 160, 238-276.
- R. Lenz, Ann. Phys. Chem. (Pogg.) 1877, 160, 425-435.
- R. Lenz, https://www.biodiversitylibrary.org/item/104844#page/1/mode/1up 1877, 23, 250-279.
- The Fundamental Laws of Electrolytic Conduction. Memoirs by Faraday, Hittorf and F. Kohlrausch, Vol. VII, Harper & Brothers, New York, London, 1899.
- F.-J. Malaguti, Lec?ons E?le?mentaires de Chimie, 1. ed., Dezobry, E. Magdaleine et Co., Paris, 1853.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1879, 6, 145-210.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1879, 6, 1-51.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1885, 26, 161-226.
- F. Kohlrausch, M. E. Maltby, Wiss. Abhandl. Physik.-Techn. Reichsanstalt 1900, 3, 156-227.
- F. Kohlrausch, H. v. Steinwehr, Berlin. Ber. 1902, 581-587.
- F. Kohlrausch, Z. Elektrochem. 1907, 13, 333-344.
- R. Lenz, Mém. Acad. Impér. Sci. St.-Petersbourg 1879, 26, 1-51.
- R. Lenz, Mém. Acad. Impér. Sci. St.-Petersbourg 1882, 30, 1-64.
- O. J. Lodge, Brit. Assoc. Adv. Sci. Rep. 1886, 56, 389-413.
- W. C. D. Whetham, Phil. Mag. (5. Ser.) 1894, 38, 392-396.
- O. Masson, Phil. Trans. 1899, 192, 331-350.
- A. A. Noyes, Z. phys. Chem. 1901, 36, 63-83.
- B. D. Steele, R. B. Denison, Trans. Chem. Soc. 1902, 81, 456-469.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1897, 62, 209-239.
- F. Kohlrausch, O. Grotrian, Phil. Mag. 1875, 49, 417-425.
- A. Paalzow, Monatsber. Akad. Berlin 1868, July, 486.
- A. Paalzow, Ann. Phys. Chem. (Pogg.) 1869, 136, 489-494.
- J. H. Long, Ann. Phys. Chem. (N. F., Wied.) 1880, 11, 37-46.
- E. Bouty, Journ. Phys. 1884, 3 (2. sér.), 325.
- E. Bouty, Compt. rend. 1884, 99, 30-32.
- E. Bouty, Compt. rend. 1884, 98, 140-142.
- S. Arrhenius, Recherches sur la conductibilité galvanique des électrolytes. Première partie. La conductibilité des solutions aqueuses extremement diluées déterminée au moyen du dépolarisateur, Vol. 8, Kongl. Boktryckeriet. P. A. Norstedt & Söner, Stockholm, 1884.
- S. Arrhenius, Recherches sur la conductibilité galvanique des électrolytes. Seconde partie. Théorie chimique des électrolyte, Vol. 8, Kongl. Boktryckeriet. P. A. Norstedt & Söner, Stockholm, 1884.
- W. Ostwald, J. prakt. Chem. (N. F., Kolbe, Meyer) 1884, 30, 225-237.
- de.wikipedia.org/wiki/Friedrich_Kohlrausch_(Physiker)#/media/, Datei:Kohlrausch_et_al_WS1886-87_W%C3%BCtzburg.jpg.
- S. Arrhenius, Z. phys. Chem. 1887, 1, 631–649.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1891, 44, 577-622.
- F. Kohlrausch, Ber. Dt. Chem. Gesellsch. 1893, 26, 2998-3003.
- F. Kohlrausch, A. Heydweiller, Ann. Chem. Phys. (N. F., Wied.) 1894, 53, 209-235.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1893, 50, 385-408.
- F. Kohlrausch, W. Hallwachs, Ann. Phys. Chem. (N. F., Wied.) 1894, 53, 14-42.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1893, 49, 225-256.
- F. Kohlrausch, Z. phys. Chem. 1894, 15, 126-180.
- F. Kohlrausch, A. Heydweiller, Ann. Phys. Chem. (N. F., Wied.) 1895, 54, 385-395.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1896, 58, 514-516.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1895, 56, 185-200.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1895, 56, 177-184.
- F. Kohlrausch, L. Holborn, H. Diesselhorst, Ann. Phys. Chem. (N. F., Wied.) 1898, 64, 417-455.
- F. Kohlrausch, Verhandl. Dt. phys. Gesellsch. 1896, 126.
- F. Kohlrausch, Ann. Phys. Chem. (N. F., Wied.) 1897, 60, 315-332.
- S. H. Christie, Phil. Trans. 1833, 123, 95-142.
- C. Wheatstone, Phil. Trans. 1843, 133, 303-327.
- F. Kohlrausch, A. Heydweiller, Z. phys. Chem. 1894, 14, 317-330.
- M. Rudolphi, Z. phys. Chem. 1895, 17, 385-426.
- J. H. van ´t Hoff, Z. phys. Chem. 1895, 18, 300-304.
- F. Kohlrausch, Z. phys. Chem. 1910, 72, 43-48.
- F. Kohlrausch, Elektrotechn. Z. 1887, 8, 258-265.
- H. Buff, Ann. Chem. Pharm. 1858, 105, 145-176.
- W. Ostwald, Z. phys. Chem. 1888, 2, 270–283.
- W. Ostwald, W. Nernst, Z. phys. Chem. 1889, 3, 120-130.
- G. Lippmann, Ann. Chim. Phys 1875, 5 (Ser. 5), 494-549.
- E. Becquerel, Annales du conservatoire impériál des arts et des métiers 1861, 1, 734-754.
- W. Beetz, Ann. Phys. Chem. (Pogg.) 1865, 125, 126-132.
- C. Blondel, in Les professeurs du Conservatoire National des Arts et Métiers. Dictionnaire biographique 1794-1955, Vol. 1 (A - K), Institut national de recherche pédagogique, Paris, 1994, pp. 168-182.
- E. Becquerel, Ann. Chim. Phys. 1846, 17 (3. sér.), 242-290.
- E. Becquerel, Ann. Phys. Chem. (Pogg.) 1847, 70, 238-254.
- E. Becquerel, Ann. Chim. Phys. 1847, 20 (3. sér.), 53-84.
- A. D. C. I. D. A. E. D. MÉTIERS, Annales du conservatoire impériál des arts et des métiers, Vol. 1, E. Lacroix, Paris, 1861.
- R. Bunsen, Ann. Chem. Pharm. (Wöh., Lieb.) 1841, 38, 311–313.
- R. Bunsen, Ann. Phys. Chem. (Pogg.) 1841, 54, 417–430.
- L. Onsager, Physik. Z. 1926, 27, 388-392.
- L. Onsager, Physik. Z. 1927, 28, 277-298.
- P. Debye, E. Hückel, Physik. Z. 1923, 24, 305-325.
- P. Debye, E. Hückel, Physik. Z. 1923, 24, 185-206.





