Published 2022-03-07
Keywords
- Pharmacology,
- Alzheimer,
- psychiatry,
- cancer,
- entropy
- pH,
- mitochondria,
- lactic acid,
- paradigm shift ...More
How to Cite
Copyright (c) 2022 Laurent Schwartz, Luc Benichou, Khalid Omer Alfarouk, Marc Henry

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
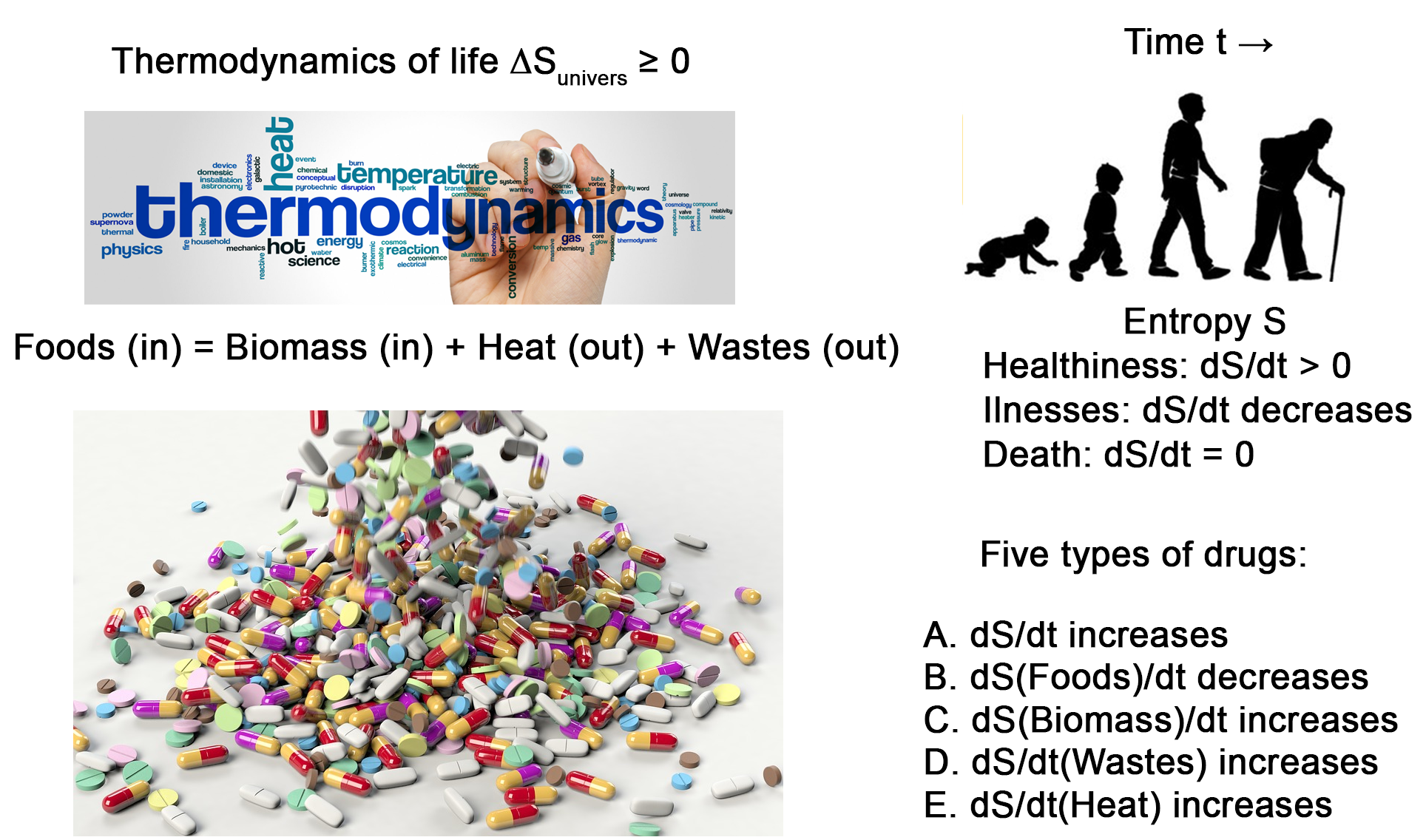
Specialization characterizes pharmacology, with the consequence of classifying the various treatments into unrelated categories. Treating a specific disease usually requires the design of a specific drug. The second law of thermodynamics is the driving force both for chemical reactions and for life. It applies to diseases and treatment. In most common diseases, there is a metabolic shift toward anabolism and anaerobic glycolysis, resulting in the release of entropy in the form of biomass. In accordance with the second principle of thermodynamics, treatment should aim at decreasing the entropy flux, which stays inside the body in the form of biomass. Most treatments aim at increasing the amount of entropy that is released by the cell in the form of thermal photons. As clinically different diseases often requires similar drugs, this calls for reinforcement in a quest for a single unified framework. For example, treatment of aggressive autoimmune diseases requires the same cytotoxic chemotherapy than for cancer. This strongly suggests that despite their apparent disparity, there is an underlying unity in the diseases and the treatments. The shift toward increased entropy release in the form of heat offers sound guidelines for the repurposing of drugs.
References
- Seyfried, T.N.; Flores, R.E.; Poff, A.M., D’Agostino, D.P. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis 2014, 35, 515–27, doi:10.1093/carcin/bgt480.
- Da Veiga Moreira, J.; Peres, S.; Steyaert, J.-M.M.; Bigan, E.; Paulevé, L.; Nogueira, M.L.; Schwartz, L. Cell cycle progression is regulated by intertwined redox oscillators. Theor. Biol. Med. Model. 2015, 12, 10, doi:10.1186/s12976-015-0005-2.
- Schwartz, L.; Peres, S.; Jolicoeur, M.; da Veiga Moreira, J. Cancer and Alzheimer’s disease: intracellular pH scales the metabolic disorders. Biogerontology 2020, 21(6), 683-694.doi: 10.1007/s10522-020-09888-6.
- Schwartz L.; Henry M.; Alfaroukh K.O.; Reshkin, S. J.; Radman M., Metabolic shifts as the hallmark of most common diseases: the quest for underlying unity. International Journal of Molecular Science, 2021, 22, 3972. doi: 10.3390/ijms22083972
- Zimmerman, A.W.; Jyonouchi, H.; Comi, A.M.; Connors, S.L.; Milstien, S.; Varsou, A., Heyes, M.P. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr. Neurol. 2005, 33, 195–201, doi:10.1016/j.pediatrneurol.2005.03.014.
- Vallée, A.; Vallée, J.N. Warburg effect hypothesis in autism Spectrum disorders. Mol. Brain 2018, 11 (1), 1. doi: 10.1186/s13041-017-0343-6
- Looney, J.M.; Childs, H.M. The lactic acid and glutathione content of the blood of schizophrenic patients. J. Clin. Invest. 1934, 13, 963–968, doi:10.1172/jci100639.
- Kösel, S.; Hofhaus, G.; Maassen, A.; Vieregge, P.; Graeber, M.B. Role of mitochondria in Parkinson disease. Biol. Chem. 1999, 380, 865–870. doi: 10.1515/BC.1999.106
- Yamamoto, M.; Ujike, U.; Wada, K.; Tsuji, T. Cerebrospinal fluid lactate and pyruvate concentrations in patients with Parkinson’s disease and mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS). J. Neurol. Neurosurg. Psychiatry 1997, 62, doi:10.1136/JNNP.62.3.290.
- Quintanilla, R.A.; Jin, Y.N.; Von Bernhardi, R.; Johnson, G.V. Mitochondrial permeability transition pore induces mitochondria injury in Huntington disease. Mol. Neurodegener. 2013, 8, 45–45, doi:10.1186/1750-1326-8-45.
- Sims, N.R.; Muyderman, H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim. Biophys. Acta - Mol. Basis Dis. 2010, 1802, 80–91. doi: 10.1016/j.bbadis.2009.09.003
- Bruhn, H.; Frahm, J.; Gyngell, M.L.; Merboldt, K.D.; Hänicke, W.; Sauter, R. Cerebral metabolism in man after acute stroke: New observations using localized proton NMR spectroscopy. Magn. Reson. Med. 1989, 9, 126–131, doi:10.1002/mrm.1910090115.
- Yamada, H.; Chounan, R.; Higashi, Y.; Kurihara, N.; Kido, H. Mitochondrial targeting sequence of the influenza A virus PB1-F2 protein and its function in mitochondria. FEBS Lett. 2004, 578, 331–336, doi:10.1016/j.febslet.2004.11.017.
- Yu, G.; Tzouvelekis, A.; Wang, R.; Herazo-Maya, J.D.; Ibarra, G.H.; Srivastava, A.; De Castro, J.P.W.; Deiuliis, G.; Ahangari, F.; Woolard, T.; et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018, 24, 39–49, doi:10.1038/nm.4447.
- Maher, T.M. Aerobic glycolysis and the Warburg effect an unexplored realm in the search for fibrosis therapies? Am. J. Respir. Crit. Care Med. 2015, 192, 1407–1409. doi: 10.1164/rccm.201508-1699ED
- Krähenbühl, S.; Stucki, J.; Reichen, J. Reduced activity of the electron transport chain in liver mitochondria isolated from rats with secondary biliary cirrhosis. Hepatology 1992, 15, 1160–1166, doi:10.1002/hep.1840150630.
- Kershenobich, D.; García-Tsao, G.; Saldana, S.A.; Rojkind, M. Relationship between blood lactic acid and serum proline in alcoholic liver cirrhosis. Gastroenterology 1981, 80, 1012–1015, doi:10.1016/0016-5085(81)90074-3
- Morrison, W.L.; Gibson, J.N.A.; Scrimgeour, C.; Rennie, M.J. Muscle wasting in emphysema. Clin. Sci. 1988, 75, 415–420, doi:10.1042/cs0750415.
- Mitsunaga, S.; Hosomichi, K.; Okudaira, Y.; Nakaoka, H.; Suzuki, Y.; Kuwana, M.; Sato, S.; Kaneko, Y.; Homma, Y.; Oka, A.; et al. Aggregation of rare/low-frequency variants of the mitochondria respiratory chain-related proteins in rheumatoid arthritis patients. J. Hum. Genet. 2015, 60, 449–454, doi:10.1038/jhg.2015.50.
- Reimer, G. Autoantibodies against nuclear, nucleolar, and mitochondrial antigens in systemic sclerosis (scleroderma). Rheum. Dis. Clin. North Am. 1990, 16, 169–183. PMID: 2406806
- Yang, S.-K.; Zhang, H.-R.; Shi, S.-P.; Zhu, Y.-Q.; Song, N.; Dai, Q.; Zhang, W.; Gui, M.; Zhang, H. The Role of Mitochondria in Systemic Lupus Erythematosus: A Glimpse of Various Pathogenetic Mechanisms. Curr. Med. Chem. 2018, 27, 3346–3361, doi:10.2174/0929867326666181126165139
- McCaffrey, L.M.; Petelin, A.; Cunha, B.A. Systemic lupus erythematosus (SLE) cerebritis versus Listeria monocytogenes meningoencephalitis in a patient with systemic lupus erythematosus on chronic corticosteroid therapy: The diagnostic importance of cerebrospinal fluid (CSF) of lactic acid levels. Hear. Lung J. Acute Crit. Care 2012, 41, 394–397, doi:10.1016/j.hrtlng.2011.09.002.
- . Henry, M.; Schwartz, L. Entropy export as the driving force of evolution. Substantia 2019, 3, 29–56. https://doi.org/10.13128/Substantia-324
- Schwartz, L.; Devin, A.; Bouillaud, F.; Henry, M. Entropy as the Driving Force of Pathogenesis: an Attempt of Diseases Classification Based on the Laws of Physics. Substantia 2020, 4(2), 7-13, doi:10.13128/Substantia-865.
- Henry, M. (2021). Thermodynamics of Life. Substantia 2021, 5(1), 43-71. https://doi.org/10.36253/Substantia-959
- Lehninger, A.L. Bioenergetics: The Molecular Basis of Biological Energy Transformations; Benjamin-Cummings Publishing Compan, 1965.
- Schwartz, L.; da Veiga Moreira, J.; Jolicoeur, M. Physical forces modulate cell differentiation and proliferation processes. J. Cell. Mol. Med. 2018, 22, 738–745. doi: 10.1111/jcmm.13417
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More Than Just a Powerhouse. Curr. Biol. 2006, 16. doi: 10.1016/j.cub.2006.06.054
- Laflaquière, B.; Leclercq, G.; Choey, C.; Chen, J.; Peres, S.; Ito, C.; Jolicoeur, M. Identifying biomarkers of Wharton’s Jelly mesenchymal stromal cells using a dynamic metabolic model: The cell passage effect. Metabolites 2018, 8, doi:10.3390/metabo8010018.
- Moreira, J. da V.; Hamraz, M.; Abolhassani, M.; Bigan, E.; Pérès, S.; Paulevé, L.; Nogueira, M.L.; Steyaert, J.-M.; Schwartz, L. The Redox Status of Cancer Cells Supports Mechanisms behind the Warburg Effect. Metabolites 2016, 6 doi:10.3390/metabo6040033.
- Chiche, J.; Ilc, K.; Laferrière, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009, 69, 358–368, doi:10.1158/0008-5472.CAN-08-2470.
- McCully, K.K.; Fielding, R.A.; Evans, W.J.; Leigh, J.S.; Posner, J.D. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J. Appl. Physiol. 1993, 75, 813–819, doi:10.1152/jappl.1993.75.2.813.
- Warrell, D. A.; Benz Jr, E. J.; Cox, T. M.; Firth, J. D. (Eds.). (2003). Oxford textbook of medicine. Oxford University Press, USA.
- Goglia, F.; Silvestri, E.; Lanni, A. Thyroid hormones and mitochondria. Bioscience reports 2002, 22(1), 17-32. doi: 10.1023/a:1016056905347
- Ricquier, D.; Bouillaud, F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. The Journal of physiology 2000, 529(1), 3-10. doi: 10.1111/j.1469-7793.2000.00003.x
- Barbosa, D.J.; Capela, J.P.; Feio-Azevedo, R.; Teixeira-Gomes, A.; de Lourdes Bastos, M.; Carvalho, F. Mitochondria key players in the toxicity of the amphetamines. Archives in toxicology 2015, 89, 1695-1725. doi: 10.1007/s00204-015-1478-9
- Brown, J. M.; Yamamoto, B. K. Effects of amphetamines on mitochondrial function: role of free radicals and oxidative stress. Pharmacology & therapeutics 2003, 99(1), 45-53.
- Tsygani?, A. A.; Medvinskaia, N. A.; Rudenko, A. F. Effect of digoxin, strophanthin and isolanid on oxygen absorption, oxidative phosphorylation and the amount of cytochromes in the myocardial mitochondria and their ATPase activity. Farmakologiia i toksikologiia, 1982, 45(1), 30-32.
- Platz, E. A.; Yegnasubramanian, S.; Liu, J. O.; Chong, C. R.; Shim, J. S.; Kenfield, S. A.; Nelson, W. G. A novel two-stage, transdisciplinary study identifies digoxin as a possible drug for prostate cancer treatment. Cancer discovery 2011, 1(1), 68-77. doi: 10.1158/2159-8274.CD-10-0020
- Balestra, G. M.; Mik, E. G.; Eerbeek, O.; Specht, P. A.; Van der Laarse, W. J.; Zuurbier, C. . Increased in vivo mitochondrial oxygenation with right ventricular failure induced by pulmonary arterial hypertension: mitochondrial inhibition as driver of cardiac failure?. Respiratory research 2015, 16(1), 1-10. doi: 10.1186/s12931-015-0178-6
- Colman, R. J.; Anderson, R. M.; Johnson, S. C.; Kastman, E. K.; Kosmatka, K. J.; Beasley, T. M., Weindruch, R. (2009). Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 2009, 325(5937), 201-204. doi: 10.1126/science.1173635
- Filiou, M.D.; Sandi, C. Anxiety and Brain Mitochondria: A Bidirectional Crosstalk Trends Neurosci 201), 42, 573–588. doi: 10.1016/j.tins.2019.07.002
- Lin, S.; Jin, P.; Shao, C.; Lu, W.; Xiang, Q., Jiang, Z., Bian, J. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses and protects against endotoxemia in mice by suppressing HIF1?-induced glycolysis. International immunopharmacology 2020, 80, 106-150.
- Schwartz, D.; Beitner, R. Detachment of the glycolytic enzymes, phosphofructokinase and aldolase, from cytoskeleton of melanoma cells, induced by local anesthetics. Molecular genetics and metabolism 2020, 69(2), 159-164. doi: 10.1006/mgme.2000.2960
- Drewes, L. R.; Gilboe, D. D. Glycolysis and the permeation of glucose and lactate in the isolated, perfused dog brain during anoxia and postanoxic recovery. Journal of Biological Chemistry 1973, 248(7), 2489-2496.
- Gey, K. F.; Rutishauser, M.; Pletscher, A. Suppression of glycolysis in rat brain in vivo by chlorpromazine, reserpine, and phenobarbital. Biochemical pharmacology 1965, 14(4), 507-514. doi: 10.1016/0006-2952(65)90223-6
- Qi, J.; Wu, Q.; Zhu, X., Zhang, S.; Chen, X.; Chen, W; Sun, Z.; Zhu, M.; Miao, C. Propofol attenuates the adhesion of tumor and endothelial cells through inhibiting glycolysis in human umbilical vein endothelial cells. Acta biochimica et biophysica Sinica 2019, 51(11), 1114-1122. doi: 10.1093/abbs/gmz105
- Uehara, T.; Sumiyoshi, T.; Matsuoka, T.; Tanaka, K.; Tsunoda, M.; Itoh, H.; Kurachi, M. Enhancement of lactate metabolism in the basolateral amygdala by physical and psychological stress: role of benzodiazepine receptors. Brain research 2005, 1065(1-2), 86-91. doi: 10.1016/j.brainres.2005.10.035
- Greengard, O.; McIlwain, H. Anticonvulsants and the metabolism of separated mammalian cerebral tissues. Biochemical Journal 1955, 61(1), 61-8. doi: 10.1042/bj0610061.
- Shao, L. R.; Rho, J. M.; Stafstrom, C. E. Glycolytic inhibition: A novel approach toward controlling neuronal excitability and seizures. Epilepsia Open 2018, 3, 191-197. doi: 10.1002/epi4.12251
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood lactate measurements and analysis during exercise: A guide for clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. DOI: 10.1177/193229680700100414
- Roth, W.T.; Gomolla, A.; Meuret, A.E.; Alpers, G.W.; Handke, E.M.; Wilhelm, F.H. High altitudes, anxiety, and panic attacks: Is there a relationship? Depress. Anxiety 2002, 16, 51–58. DOI: 10.1002/da.10059
- Dratcu, L. Panic, hyperventilation and perpetuation of anxiety. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2000, 24, 1069–1089. doi: 10.1016/s0278-5846(00)00130-5
- El-Ad, B.; Lavie, P. Effect of sleep apnea on cognition and mood. Int. Rev. Psychiatry 2005, 17, 277–282. doi: 10.1080/09540260500104508
- Pitts, F.N.; McClure, J.N. Lactate metabolism in anxiety neurosis. N. Engl. J. Med. 1967, 277, 1329–1336. doi: 10.1056/NEJM196712212772502
- Liebowitz, M.R.; Hollander, E. Lactate-induced anxiety. Biol. Psychiatry 1989, 25, 669–670. doi: 10.1016/0006-3223(89)90235-7
- Hollander, E.; Liebowitz, M.R.; Gorman, J.M.; Cohen, B.; Fyer, A.; Klein, D.F. Cortisol and Sodium Lactate—Induced Panic. Arch. Gen. Psychiatry 1989, 46, 135-140. doi: 10.1001/archpsyc.1989.01810020037007
- Sajdyk, T. J., & Shekhar, A. (2000). Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. European journal of pharmacology, 394(2-3), 265-273. DOI: 10.1016/s0014-2999(00)00128-x
- Ziemann, A.E.; Allen, J.E.; Dahdaleh, N.S.; Drebot, I.I.; Coryell, M.W.; Wunsch, A.M.; Lynch, C.M.; Faraci, F.M.; Howard, M.A.; Welsh, M.J.; et al. The Amygdala Is a Chemosensor that Detects Carbon Dioxide and Acidosis to Elicit Fear Behavior. Cell 2009, 139, 1012–1021. doi: 10.1016/j.cell.2009.10.029
- Van Der Kooij, M.A.; Hollis, F.; Lozano, L.; Zalachoras, I.; Abad, S.; Zanoletti, O.; Grosse, J.; Guillot De Suduiraut, I.; Canto, C.; Sandi, C. Diazepam actions in the VTA enhance social dominance and mitochondrial function in the nucleus accumbens by activation of dopamine D1 receptors. Mol. Psychiatry 2018, 23, 569–578. doi: 10.1038/mp.2017.135
- Cohen, G.; Kesler, N. Monoamine oxidase and mitochondrial respiration. J. Neurochem. 1999, 73, 2310–2315. doi: 10.1046/j.1471-4159.1999.0732310.x
- Chafe, S. C.; Vizeacoumar, F. S. ; Venkateswaran, G.; Nemirovsky, O.; Awrey, S.; Brown, W. S.; McDonald, P. C.; Carta, F.; Metcalfe, A.; Karasinska, J. M.; Huang, L.; Muthuswamy, S. K.; Schaeffer, D. F.; Renouf, D. J.; Supuran, C. T.; Vizeacoumar, F. J.; Dedhar, S. Genome-wide synthetic lethal screen unveils novel CAIX-NFS1/xCT axis as a targetable vulnerability in hypoxic solid tumors. Sci Adv. 2021, Aug 27; 7(35), eabj0364.
- Fanibunda, S.E.; Deb, S.; Maniyadath, B.; Tiwari, P.; Ghai, U.; Gupta, S.; Figueiredo, D.; Weisstaub, N.; Gingrich, J.A.; Vaidya, A.D.B.; et al. Serotonin regulates mitochondrial biogenesis and function in rodent cortical neurons via the 5-HT2A receptor and SIRT1–PGC-1? axis. Proc. Natl. Acad. Sci. U. S. A. 2019, 166, 11028–11037. doi: 10.1073/pnas.1821332116
- Stanhope, K. L. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annual review of medicine, 2012 63, 329-343. doi: 10.1146/annurev-med-042010-113026
- Rumawas, M. E.; Meigs, J. B.; Dwyer, J. T.; McKeown, N. M.; Jacques, P. F. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. The American journal of clinical nutrition 2009, 90(6), 1608-1614. doi: 10.3945/ajcn.2009.27908
- Russell?Jones, D.; Khan, R. Insulin?associated weight gain in diabetes–causes, effects and coping strategies. Diabetes, Obesity and Metabolism 2007, 9(6), 799-812 doi: 10.1111/j.1463-1326.2006.00686.x
- Sjöqvist, F.; Garle, M.; Rane, A. Use of doping agents, particularly anabolic steroids, in sports and society. he Lancet 2008, 371(9627), 1872-1882. doi: 10.1016/S0140-6736(08)60801-6
- Hoffmann, R. F.; Jonker, M. R.; Brandenburg, S. M.; de Bruin, H. G.; Ten Hacken, N. H. T.; van Oosterhout, A. J. M.; Heijink, I. H. Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Scientific reports 2019, 9(1), 1-10. doi: 10.1038/s41598-019-51517-x
- Thieme, D., & Hemmersbach, P. (Eds.). (2009). Doping in sports(Vol. 195). Springer Science & Business Media.
- Roesch, D. M. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiology & behavior 2006, 87(1), 39-44. doi: 10.1016/j.physbeh.2005.08.035
- Warrell, D. A.; Benz Jr., E. J.; Cox, T. M.; Firth, J. D. (Eds.). (2003). Oxford textbook of medicine. Oxford University Press, USA.
- Chen, X.; Lu, W.; Zheng, W.; Gu, K.; Matthews, C. E.; Chen, Z.; Shu, X. O. Exercise after diagnosis of breast cancer in association with survival. Cancer Prevention Research 2011, 4(9), 1409-1418. doi: 10.1158/1940-6207.CAPR-10-0355
- Teri, L.; Gibbons, L. E.; McCurry, S. M.; Logsdon, R. G.; Buchner, D. M.; Barlow, W. E.; Kukul, W. A.; Lactoix, A.; McCormick, W.; Larson, E. B. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA 2003, 290(15), 2015-2022. doi: 10.1001/jama.290.15.2015
- Abolhassani, M.; Wertz, X.; Pooya, M.; Chaumet-Riffaud, P.; Guais, A.; Schwartz, L. Hyperosmolarity causes inflammation through the methylation of protein phosphatase 2A. Inflamm. Res. 2008, 57, 419–29, doi:10.1007/s00011-007-7213-0.
- Schwartz, L.; M. Israël, I.P.; Schwartz, L.; Israël, M.; Philippe, I. Inflammation and carcinogenesis: A change in the metabolic process. In Cancer Microenvironment and Therapeutic Implications; Baronzio, G., Fiorentini, G., Cogle, C.R., Eds.; Springer Netherlands: Dordrecht, 2009; pp. 3–18 ISBN 978-1-4020-9575-7.
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is inflammation a consequence of extracellular hyperosmolarity? J. Inflamm. (Lond). 2009, 6, 21, doi:10.1186/1476-9255-6-21.
- Hamraz, M.; Abolhassani, R.; Andriamihaja, M.; Ransy, C.; Lenoir, V.; Schwartz, L.; Bouillaud, F. Hypertonic external medium represses cellular respiration and promotes Warburg/Crabtree effect. FASEB J. 2020, 34, 222–236, doi:10.1096/fj.201900706RR.
- Kroetz, D. L.; Zeldin, D. C. (2002). Cytochrome P450 pathways of arachidonic acid metabolism. Current opinion in lipidology, 2002, 13(3), 273-283. doi: 10.1097/00041433-200206000-00007
- Kostakoglu, L.; Agress Jr, H.; Goldsmith, S. J. Clinical role of FDG PET in evaluation of cancer patients. Radiographics, 2003, 23(2), 315-340. doi: 10.1148/rg.232025705
- Flanagan, F. L.; Dehdashti, F.; Siegel, B. A. (1998, October). PET in breast cancer. In Seminars in nuclear medicine (Vol. 28, No. 4, pp. 290-302). WB Saunders.
- Dias, N.; Bailly, C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem. Pharmacol 2005, 70(1), 1-12. doi: 10.1016/j.bcp.2005.03.021
- Schwartz, L.; Abolhassani, M.; Guais, A.; Sanders, E.; Steyaert, J. M.; Campion, F.; Israël, M. A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: preliminary results. Oncology reports 2010, 23(5), 1407-1416. doi: 10.3892/or_00000778
- Alda, M. Methylene Blue in the Treatment of Neuropsychiatric Disorders. CNS Drugs 2019, 33, 719–725. doi: 10.1007/s40263-019-00641-3
- Gonzalez-Lima, F.; Barksdale, B. R.; Rojas, J. C.. Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochemical pharmacology, 2014, 88(4), 584-593. doi: 10.1016/j.bcp.2013.11.010
- Yang, S. H.; Li, W.; Sumien, N.; Forster, M.; Simpkins, J. W.; Liu, R. (2017). Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots. Progress in neurobiology, 2017, 157, 273-291. doi :10.1016/j.pneurobio.2015.10.005
- Montégut, L.; Martínez-Basilio, P. C.; da Veiga Moreira, J.; Schwartz, L.; Jolicoeur, M. Combining lipoic acid to methylene blue reduces the Warburg effect in CHO cells: From TCA cycle activation to enhancing monoclonal antibody production. PloS one 2020, 15(4), e0231770. doi: 10.1371/journal.pone.0231770
- Schwartz, L.; T Supuran, C.; Alfarouk, O. K. The Warburg effect and the hallmarks of cancer. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents 2017, 17(2), 164-170. doi: 10.2174/1871520616666161031143301
- Adzic, M.; Brkic, Z.; Bulajic, S.; Mitic, M.; Radojcic, M. B. Antidepressant action on mitochondrial dysfunction in psychiatric disorders. Drug development research 2016, 77(7), 400-406. doi: 10.1002/ddr.21332
- Bachman, E.; Zbinden, G. Effect of antidepressant and neuroleptic drugs on respiratory function of rat heart mitochondria. Biochemical pharmacology 1979, 28(24), 3519-3524. doi: 10.1016/0006-2952(79)90394-0
- Yung, C. Y. A review of clinical trials of lithium in medicine. Pharmacology Biochemistry and Behavior 1984, 21, 51-55. doi: 10.1016/0091-3057(84)90163-1
- Giat, E.; Ehrenfeld, M.; Shoenfeld, Y. Cancer and autoimmune diseases. Autoimmunity reviews 2017, 16(10), 1049-1057. doi: 10.1016/j.autrev.2017.07.022





