Published 2021-03-22
Keywords
- Ion Flotation,
- Green Surfactant,
- Chemical synthetic surfactant,
- Biosurfactant,
- Water Treatment
- Toxic heavy metals ...More
How to Cite
Copyright (c) 2020 Mohammad Ziaee, Mojtaba Taseidifar, Richard M. Pashley, Barry W. Ninham

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
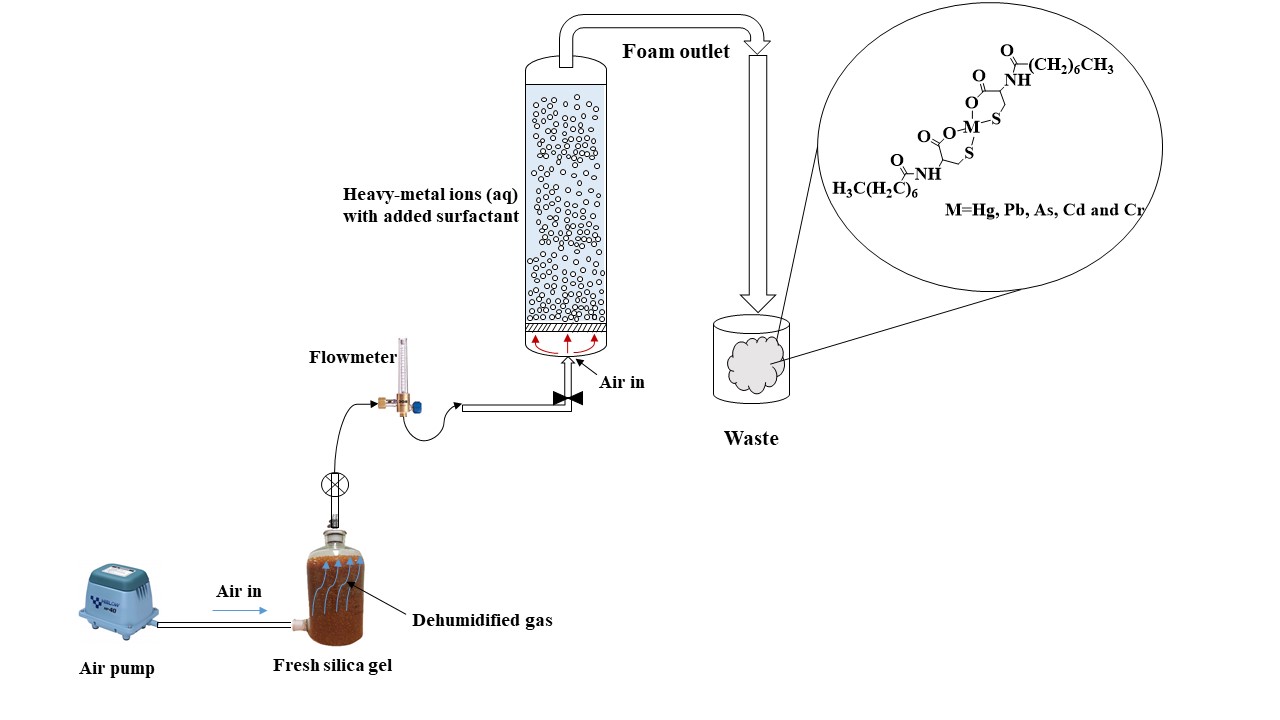
Pollution of drinking water by toxic heavy-metal ions is a matter of concern worldwide. These ions occur naturally, and also from environmental spills, radioactive wastes and other industrial waste. Arsenic and lead are typical examples. A novel green surfactant, purpose designed, and environmentally friendly is shown to be extremely effective and specific for heavy metal ion removal.
This is a considerable step forward on previous technologies. Surfactants have been used universally to remove organic and inorganic contaminants from water. But little selectivity has been achieved. After usage, the residual surfactants are discharged into surface waters or sewage systems. This causes environmental pollution.
In this review, three surfactants from different classes (novel green surfactant, synthetic chemical surfactant and biosurfactant) are compared in terms of their efficiency in flotation, removal of different heavy-metal ions, biodegradability, and toxicity level, including their advantages and disadvantages.
References
- I.Y. El-Sherif, S. Tolani, K. Ofosu, O.A. Mohamed, A.K. Wanekaya, Polymeric nanofibers for the removal of Cr(III) from tannery waste water, J. Environ. Manage., 2013, 129, 410-413.
- K. Fischer, H.-P. Bipp, Removal of Heavy Metals from Soil Components and Soils by Natural Chelating Agents. Part II. Soil Extraction by Sugar Acids, Water, Air, Soil Pollut., 2002, 138, 271-288.
- C. Mohanna, Y. Nys, Effect of dietary zinc content and sources on the growth, body zinc deposition and retention, zinc excretion and immune response in chickens, British Poultry Science, 1999, 40, 108-114.
- WHO, Copper in drinking-water, in, WHO, 2004.
- WHO, Mercury in Drinking-water, in: Background document for development of WHO Guidelines for Drinking-water Quality, 2005.
- Z. Yu, X. Zhang, Y. Huang, Magnetic Chitosan–Iron(III) Hydrogel as a Fast and Reusable Adsorbent for Chromium(VI) Removal, Ind. Eng. Chem. Res., 2013, 52, 11956-11966.
- D. Ko, J.S. Lee, H.A. Patel, M.H. Jakobsen, Y. Hwang, C.T. Yavuz, H.C.B. Hansen, H.R. Andersen, Selective removal of heavy metal ions by disulfide linked polymer networks, J. Hazard. Mater., 2017, 332, 140-148.
- C.K. Jain, I. Ali, Arsenic: Occurrence, toxicity and speciation techniques, Water Res., 2000, 34, 4304-4312.
- P.L. Smedley, D.G. Kinniburgh, A review of the source, behaviour and distribution of arsenic in natural waters, Appl. Geochem., 2002, 17, 517-568.
- M.J. Rosen, J.T. Kunjappu, Micelle Formation by Surfactants, in: Surfactants and Interfacial Phenomena, John Wiley & Sons, Inc., 2012, 123-201.
- H. Polat, D. Erdogan, Heavy metal removal from waste waters by ion flotation, J. Hazard. Mater., 2007, 148, 267-273.
- M. Taseidifar, M. Ziaee, R.M. Pashley, B.W. Ninham, Ion flotation removal of a range of contaminant ions from drinking water, J. Environ. Chem. Eng., 2019, 7(4), 103263.
- S. Rebello, A.K. Asok, S. Mundayoor, M.S. Jisha, Surfactants: toxicity, remediation and green surfactants, Environ. Chem. Lett., 2014, 12, 275-287.
- S. Shen, X.-F. Li, W.R. Cullen, M. Weinfeld, X.C. Le, Arsenic binding to proteins, Chem. Rev., 2013, 113, 7769-7792.
- M. Taseidifar, F. Makavipour, R.M. Pashley, A.F.M.M. Rahman, Removal of heavy metal ions from water using ion flotation, Environ. Technol. Innov., 2017, 8, 182-190.
- M. Taseidifar, Environmental applications of a biodegradable cysteine-based surfactant, Ecotoxicol. Environ. Saf., 2020, 206, 111389.
- B.T. Farrer, C.P. McClure, J.E. Penner-Hahn, V.L. Pecoraro, Arsenic(III)?Cysteine Interactions Stabilize Three-Helix Bundles in Aqueous Solution, Inorg. Chem., 2000, 39, 5422-5423.
- M.C. Teixeira, V.S.T. Ciminelli, M.S.S. Dantas, S.F. Diniz, H.A. Duarte, Raman spectroscopy and DFT calculations of As(III) complexation with a cysteine-rich biomaterial, J. Colloid Interface Sci., 2000, 315, 128-134.
- C. Baumann, A. Beil, S. Jurt, M. Niederwanger, O. Palacios, M. Capdevila, S. Atrian, R. Dallinger, O. Zerbe, Structural Adaptation of a Protein to Increased Metal Stress: NMR Structure of a Marine Snail Metallothionein with an Additional Domain, Angew. Chem. Int. Ed., 2017, 56, 4617-4622.
- F. Makavipour, R.M. Pashley, A.F.M.M. Rahman, Low-Level Arsenic Removal from Drinking Water, Global Challenges, 2019, 3(3), 1700047.
- J.F. Ferguson, J. Gavis, A review of the arsenic cycle in natural waters, Water Res., 1972, 6, 1259-1274.
- B. Maher, Measuring arsenic in rice – an Australian reference material, Royal Australian Chemical Institute, 2015, pp. 38.
- F. Sebba, Concentration by Ion Flotation, Nature, 1959, 184, 1062-1063.
- L.-C. Shen, X.-T. Nguyen, N.P. Hankins, Removal of heavy metal ions from dilute aqueous solutions by polymer–surfactant aggregates: A novel effluent treatment process, Sep. Purif. Technol., 2015, 152, 101-107.
- T. Cserhati, E. Forgács, G. Oros, Biological activity and environmental impact of anionic surfactants, Environ. Int., 2002, 28, 337-348.
- R. Waninge, M. Paulsson, T. Nylander, B. Ninham, P. Sellers, Binding of Sodium Dodecyl Sulphate and Dodecyl Trimethyl Ammonium Chloride to ?-Lactoglobulin: A Calorimetric Study, Int. Dairy J., 1998, 8, 141-148.
- G. McDonnell, A.D. Russell, Antiseptics and Disinfectants: Activity, Action, and Resistance, Clin. Microbiol. Rev., 1999, 12, 147-179.
- R.B. Ashman, B.W. Ninham, Immunosuppressive effects of cationic vesicles, Mol. Immunol., 1985, 22, 609-612.
- R.B. Ashman, R.V. Blanden, B.W. Ninham, D.F. Evans, Interaction of amphiphilic aggregates with cells of the immune system, Immunol. Today, 1986, 7, 278-283.
- P. Lo Nostro, B.W. Ninham, A. Lo Nostro, G. Pesavento, L. Fratoni, P. Baglioni, Specific ion effects on the growth rates of Staphylococcus aureusandPseudomonas aeruginosa, Phy. Biol., 1986, 2, 1-7.
- B.W. Ninham, K. Larsson, P. Lo Nostro, Two sides of the coin. Part 1. Lipid and surfactant self-assembly revisited, Colloids Surf. B: Biointerfaces, 2017, 152, 326-338.
- B.W. Ninham, K. Larsson, P. Lo Nostro, Two sides of the coin. Part 2. Colloid and surface science meets real biointerfaces, Colloids Surf. B: Biointerfaces, 2017, 159, 394-404.
- T. Cserhati, Alkyl Ethoxylated and Alkylphenol Ethoxylated Nonionic Surfactants: Interaction with Bioactive Compounds and Biological Effects, Environ. Health Perspect., 1995, 103, 358-364.
- L. Chang, Y. Cao, G. Fan, C. Li, W. Peng, A review of the applications of ion floatation: wastewater treatment, mineral beneficiation and hydrometallurgy, RSC Adv., 2019, 9, 20226-20239.
- X.-L. Yu, Y. He, Development of a Rapid and Simple Method for Preparing Tea-Leaf Saponins and Investigation on Their Surface Tension Differences Compared with Tea-Seed Saponins, Molecules, 2018, 23(7), 1796.
- X.Z. Yuan, Y.T. Meng, G.M. Zeng, Y.Y. Fang, J.G. Shi, Evaluation of tea-derived biosurfactant on removing heavy metal ions from dilute wastewater by ion flotation, Colloids Surf., A:Physiochem. Eng. Aspects, 2008, 317, 256-261.
- M. Do?utan Yenidünya, Recovery of Zn(II), Mn(II) and Cu(II) in Aqueous Solutions by Foam Fractionation with Sodium Dodecyl Sulphate in Combination with Chelating Agents, Sep. Sci. Technol., 2006, 41, 1741-1756.
- V.S.H. Khoshdast, Efficient chromium removal from aqueous solutions by precipitate flotation using rhamnolipid biosurfactants, Physicochem. Probl. Miner. Process., 2018, 54, 1014–1025.
- Ü. Yenial, G. Bulut, Examination of flotation behavior of metal ions for process water remediation, J. Mol. Liq., 2017, 241, 130-135.
- A.I. Zouboulis, K.A. Maris, Removal of cadmium from dilute solutions by flotation, Water Sci. Technol., 1995, 31, 315-326.
- F.S. Hoseinian, M. Irannajad, A.J. Nooshabadi, Ion flotation for removal of Ni(II) and Zn(II) ions from wastewaters, Int. J. Miner. Process., 2015, 143, 131-137.
- T. Pekdemir, S. Tokunaga, Y. Ishigami, K.-J. Hong, Removal of cadmium or lead from polluted water by biological amphiphiles, J Surfactants Deterg., 2000, 3, 43-46.
- J. Tang, J. He, X. Xin, H. Hu, T. Liu, Biosurfactants enhanced heavy metals removal from sludge in the electrokinetic treatment, Chem. Eng. J., 2018, 334, 2579-2592.
- S. Shekhar, A. Sundaramanickam, T. Balasubramanian, Biosurfactant Producing Microbes and their Potential Applications: A Review, Crit. Rev. Environ. Sci. Technol., 2015, 45, 1522-1554.
- E.Z. Ron, E. Rosenberg, Natural roles of biosurfactants, Environ. Microbiol., 2001, 3, 229-236.
- C.N. Mulligan, Recent advances in the environmental applications of biosurfactants, Curr. Opin. Colloid Interface Sci., 2009, 14, 372-378.
- R. Cohen, D. Exerowa, Surface forces and properties of foam films from rhamnolipid biosurfactants, Adv. Colloid Interface Sci., 2007, 134-135, 24-34.
- M. Taseidifar, A.G. Sanchis, R.M. Pashley, B.W. Ninham, Novel water treatment processes, Substantia, 2019, 3(2), 11-17.
- H. K. Kim, E. Tuite, B. Nordén, B. W. Ninham, Co-ion dependence of DNA nuclease activity suggests hydrophobic cavitation as a potential source of activation energy, Eur. Phys. J., 2001, 4, 411-417.




