Controlled Growth of Strontium Sulfate Particles in Aqueous Solution: Inhibition Effects of a Bubble Column Evaporator
Published 2021-03-22
Keywords
- Strontium sulfate,
- aqueous precipitation,
- nanobubbles,
- supersaturation,
- bubble column evaporator
- particle growth rates,
- crystallisation,
- bubble interaction,
- mineral flotation ...More
How to Cite
Copyright (c) 2020 Atikah Wan Nafi, Mojtaba Taseidifar, Richard M. Pashley, Barry W. Ninham

This work is licensed under a Creative Commons Attribution 4.0 International License.
Abstract
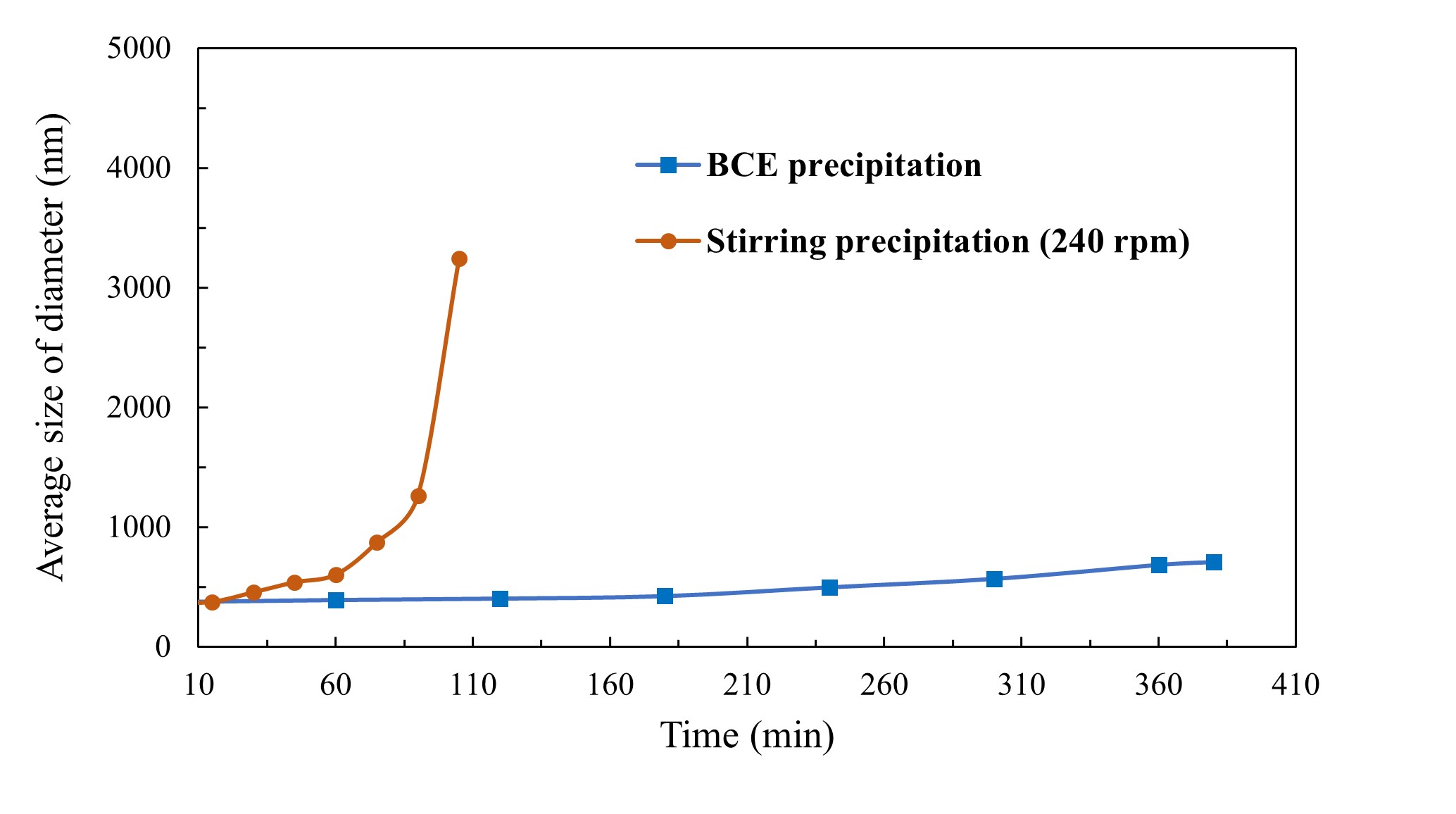
In the oil industry, strontium sulfate (SrSO4) scale deposits have long plagued oilfield and gas production operations. This remains an unsolved problem. We here show how the bubble column evaporator (BCE) can be used to control aqueous precipitation from salt solutions.
Mixtures of strontium nitrate and sodium sulfate in the BCE system were used to precipitate strontium sulfate at different degrees of supersaturation. The effectiveness of the BCE system was compared to standard mechanical stirring. The precipitation of strontium sulfate in both processes was monitored through turbidimeter, particle counting, Dynamic Light Scattering (DLS) and Scanning Electron Microscopy (SEM). The results show that the BCE system has a significant inhibition effect and so can be used to control precipitation growth rate, even from supersaturated solutions. This remarkable effect also provides new insights into mechanisms of crystallisation, of bubble interactions and mineral flotation.
References
- Z. Amjad, J. Albright, Strontium Sulfate Inhibition by Biopolymers and Synthetic Polymers, Mater. Performance, 2015, 54(12), 54-58.
- Y.D. Yeboah, M.R. Saeed, A.K. Lee, Kinetics of strontium sulfate precipitation from aqueous electrolyte solutions, J. Cryst. growth, 1994, 135(1-2), 323-330.
- F.H. Butt, F. Rahman, U. Baduruthamal, Evaluation of SHMP and advanced scale inhibitors for control of CaSO4, SrSO4, and CaCO3 scales in RO desalination, Desalination, 1997, 109(3), 323-332.
- H.M. Ezuber, Prediction of Strontium Sulfate Scale Formation in Oilfield Environment, J. ASTM Int., 2007, 4(6), 1-11.
- C.C. Patton, Applied water technology, 1986.
- A.J. Essel, B.L. Carlberg, Strontium sulfate scale control by inhibitor squeeze treatment in the Fateh field, J. Pet. Technol., 1982, 34(06), 1-302.
- J.C. Lindlof, K.G. Stoffer, A case study of seawater injection incompatibility, J. Pet. Technol., 1983, 35(07), 1-256.
- M. Nassivera, A. Essel, Fateh field sea water injection-water treatment, corrosion, and scale control, In Middle East Technical Conference and Exhibition, Society of Petroleum Engineers, 1979.
- M.S.H. Bader, Sulfate removal technologies for oil fields seawater injection operations, J. Pet. Sci. Eng., 2007, 55(1-2), 93-110.
- Z. Dai, A.T. Kan, F. Zhang, F. Yan, G. Ruan, N. Bhandari, M.B. Tomson, A Thermodynamic Model for The Solution Density and Mineral Solubility Predictions up to 250?, 1,500 Bars for Na-K-Mg-Ca-Ba-Sr-Cl-CO3-HCO3-SO4-CO2 aq Systems. In SPE International Oilfield Scale Conference and Exhibition. Society of Petroleum Engineers.
- F. Yan, F. Zhang, N. Bhandari, L. Wang, Z. Dai, Z. Zhang, M. Tomson, Adsorption and precipitation of scale inhibitors on shale formations, J. Pet. Sci. Eng., 2015, 136, 32-40.
- C. Yan, A.T. Kan, W. Wang, F. Yan, L. Wang, M.B. Tomson, Sorption Study of Al-O (OH) Nanoparticle-Crosslinked Polymeric Scale Inhibitors and Their Improved Squeeze Performance in Porous Media. SPE Journal, 2014, 19(04), 687-694.
- R.W. Howarth, A. Ingraffea, T. Engelder, Natural gas: Should fracking stop?, Nature, 2011, 477(7364), 271.
- J. S. Al-Thuwaini, B. J. Burr, Encapsulated scale inhibitor treatment, In Middle East Oil Show and Conference. Society of Petroleum Engineers, 1997.
- A.B.B. Merdhah, A.A.M. Yassin, Calcium and Strontium Sulfate Scale Formation Due to Incompatible Water, P. Int. Graduate Eng. Sci. (IGCES'08), 2008, 23, 24.
- J.D. Cogan, The Removal of Barium, Strontium, Calcium and Magnesium from Hydraulic Fracturing Produced Water Using Precipitation with Traditional and Alternative Reactant Feedstocks (Doctoral dissertation, Ohio University), 2016.
- L.O. Paugh, Marcellus shale water management challenges in Pennsylvania. In SPE Shale Gas Production Conference, Society of Petroleum Engineers, 2008.
- A. Taha, M. Amani, Water Chemistry in Oil and Gas Operations: Scales Properties and Composition, Int. J. Org. Chem., 2019, 9(3), 130-141.
- J. Sohaili, H.S. Shi, N.H. Zardari, N. Ahmad, S.K. Muniyandi, Removal of scale deposition on pipe walls by using magnetic field treatment and the effects of magnetic strength, J. Clean. Prod., 2016, 139, 1393-1399.
- J. Pichtel, Oil and gas production wastewater: Soil contamination and pollution prevention, Appl. Env. Soil Sci., 2016.
- D. Place, Water Treatment Polymers, 2017.
- R. Bahar, M.N.A. Hawlader, Desalination: conversion of seawater to freshwater, Energy (kWh/m3 4), 2013, 9(1.8), 1-8.
- W. Mark, The Guidebook to Membrane Desalination Technology: Reverse Osmosis, Nanofiltration and Hybrid Systems Process, Design, Applications and Economic. L’Aquila Desalination Publications, 2007.
- A. Ozverdi, M. Erdem, Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide, J. Hazard. Mater., 2006, 137(1), 626-632.
- M. Shahid, C. Fan, R.M. Pashley, Insight into the bubble column evaporator and its applications, Int. Rev. Phys. Chem., 2016, 1(135), 143-185.
- C. Fan, R.M. Pashley, The controlled growth of calcium sulfate dihydrate (gypsum) in aqueous solution using the inhibition effect of a bubble column evaporator, Chem. Eng. Sci., 2016, 142, 23-31.
- M. S. Atone, S. V. Moharil, S. M. Dhopte, P. L. Muthal, V. K. Kondawar, Synthesis and characterization of SrSO4: Mo, Tb thermoluminescent phosphor. physica status solidi (a), 1999, 174(2), 521-526.
- T. Murakami, J.H. Ouyang, K. Umeda, S. Sasaki, High-temperature friction properties of BaSO4 and SrSO4 powder films formed on Al2O3 and stainless steel substrates, Mater. Sci. Eng., A, 2006, 432(1-2), 52-58.
- R. Giorgi, C. Bozzi, L. Dei, C. Gabbiani, B.W. Ninham, P. Baglioni, Nanoparticles of Mg(OH)2: synthesis and application to paper conservation, Langmuir, 2005, 21(18), 8495-8501.
- Y. Kimura, Rare earth phosphate particle and its production, Japan Kokai Tokkyo Koho, 1996, JP H08-143305.
- S.A. Khan, A. Gunther, M.A. Schmidt, K.F. Jensen, Microfluidic synthesis of colloidal silica, Langmuir, 2004, 20(20), 8604-8611.
- T. Ogihara, M. Yabuuchi, T. Yanagawa, N. Ogata, K. Yoshida, N. Nagata, U. Maeda, Preparation of monodispersed, spherical ferric oxide particles by hydrolysis of metal alkoxides using a continuous tube-type reactor, Adv. Powder Technol., 1997, 8(1), 73-84.
- M. Raudino, F. Sarri, D. Tatini, M. Ambrosi, G.D. Aloisi, B.W. Ninham, L. Dei, P.L. Nostro, The Effect of Temperature and Magnetic Field on the Precipitation of Insoluble Salts of Alkaline Earth Metals, J. Solution Chem., 2020, 49(3), 289-305.
- M. A. Salman, M. Safar, G. Al-Nuwaibit, The Effect of Magnetic Treatment on Retarding Scaling Deposition, The Online J. Sci. Technol., 2015, 5(3), 62-77.
- B. J. Mason, The nuclei of atmospheric condensation, Geofisica pura e applicata, 1957, 36(1), 9-20.
- P. A. Barata, M. L. Serrano, Salting-out precipitation of potassium dihydrogen phosphate (KDP). I. Precipitation mechanism, J. Cryst. Growth, 1996, 160(3-4), 361-369.
- D.F. Jacques, B.I. Bourland, A study of solubility of strontium sulfate, Soc. Pet. Eng. J., 1983, 23(02), 292-300.
- D. Feng, C. Aldrich, H. Tan, Treatment of acid mine water by use of heavy metal precipitation and ion exchange, Miner. Eng., 2000, 13(6), 623-642.
- M. Li, Removal of divalent cations from marcellus shale flowback water through chemical precipitation (Doctoral dissertation, University of Pittsburgh), 2011.
- T. Chen, P. Chen, H. Montgomerie, T. Hagen, R. Benvie, Q. Guo, X. Yang, Do We Need Higher Dose Scale Inhibitors to Inhibit Scale under Turbulent Conditions? Insight into Mechanisms and New Test Methodology, In SPE International Oilfield Scale Conference and Exhibition, Soc. Pet. Eng. J., 2014.
- F. Yan, N. Bhandari, F. Zhang, G. Ruan, Z. Dai, Y. Liu, M. Tomson, Scale Formation and Control Under Turbulent Conditions, In SPE International Oilfield Scale Conference and Exhibition, Soc. Pet. Eng. J., 2016.
- F. Yan, Z. Dai, G. Ruan, H. Alsaiari, N. Bhandari, F. Zhang, M. Tomson, Barite scale formation and inhibition in laminar and turbulent flow: A rotating cylinder approach, J. Pet. Sci. Eng., 2017, 149, 183-192.
- F. Yan, F. Zhang, N. Bhandari, G. Ruan, H. Alsaiari, Z. Dai, A. Kan, The Effect of Turbulence on Mineral Scale Control in Oilfield, In SPE International Conference on Oilfield Chemistry, Soc. Pet. Eng. J., 2017.
- J. W. Mullin, Crystallization, Elsevier, 2001.
- A. Hina, G.H. Nancollas, Precipitation and dissolution of alkaline earth sulfates: kinetics and surface energy, Rev. Miner. Geochem., 2000, 40(1), 277-301.
- S.K. Hamdona, S.M. Hamza, Influence of polyphosphonates on the precipitation of strontium sulfate (Celestite) from aqueous solutions, J. Taibah Uni. Sci., 2009, 2(1), 36-43.
- M. C. Van der Leeden, D. Kashchiev, G. M. Van Rosmalen, Effect of additives on nucleation rate, crystal growth rate and induction time in precipitation, J. Cryst. Growth, 1993, 130(1-2), 221-232.
- D. R. Lide, H. P. R. Fredrikse, CRC handbook of chemistry and physics, CRC Press, Boca Raton, FL. CRC handbook of chemistry and physics, 75th ed. CRC Press, Boca Raton, FL, 1994.
- W. Beckmann, Crystallization: basic concepts and industrial applications, John Wiley & Sons, 2013.
- D. F. Jacques, B. I. Bourland, A study of solubility of strontium sulfate, Soc. Pet. Eng. J., 1983, 23(02), 292-300.
- E.J. Reardon, D.K. Armstrong, Celestite (SrSO4 (s)) solubility in water, seawater and NaCl solution, Geochim. Cosmochim. Acta, 1987, 51(1), 63-72.
- R. D. Howell, K. Raju, G. Atkinson, Thermodynamics of "scale" mineral solubilities. 4. Experimental measurements of strontium sulfate (s) in water and aqueous sodium chloride from 25 to 250? and from 1 to 500 bar, J. Chem. Eng. Data, 1992, 37(4), 464-469.
- V.S.J. Craig, B.W. Ninham, R.M. Pashley, Effect of electrolytes on bubble coalescence, Nature, 1993, 364(6435), 317-319.
- V. S. J. Craig, B. W. Ninham, R. M. Pashley, The effect of electrolytes on bubble coalescence in water, J. Phys. Chem., 1993, 97(39), 10192-10197.
- N. Brown, Particle size analysis of large, transparent alumina trihydrate particles by turbidimetry, Powder Technol., 1971, 4(4), 232-234.
- S. He, J. E. Oddo, M. B. Tomson, The Nucleation kinetics of strontium sulfate in NaCl solutions up to 6 m and 90 C with or without inhibitors, J. Colloid Interface Sci., 1995, 174(2), 327-335.
- W. He, J. Nan, Study on the impact of particle size distribution on turbidity in water, Desalination and Water Treat., 2012, 41(1-3), 26-34.
- I. X. Malollari, P. G. Klepetsanis, P. G. Koutsoukos, Precipitation of strontium sulfate in aqueous solutions at 25?, J. Cryst. Growth, 1995, 155(3-4), 240-246.
- A. E. Nielsen, Homogeneous nucleation in barium sulfate precipitation, Acta Chemica Scandinavica, 1961, 15(2), 441.
- A. E. Austin, J. F. Miller, N. A. Richard, J. F. Kircher, Precipitation of calcium sulfate from sea water at high temperatures, Desalination, 1975, 16(3), 331-344.
- Z. Amjad, Environment Treatment & Control MP, 1989.
- F. H. Butt, F. Rahman, U. Baduruthamal, Evaluation of SHMP and advanced scale inhibitors for control of CaSO4, SrSO4, and CaCO3 scales in RO desalination, Desalination, 1997, 109(3), 323-332.
- G. R. Campbell, G.H. Nancollas, Crystallization and dissolution of strontium sulfate in aqueous solution, J. Phy. Chem., 1969, 73(6), 1735-1740.
- M. Kawase, K. Miura, Fine particle synthesis by continuous precipitation using a tubular reactor, Adv. Powder Technol., 2007, 18(6), 725-738.
- J. Sun, R. Sun, Z. Xia, H. Du, Facile room temperature morphology-controlled synthesis of SrSO4 microcrystals, Cryst. Eng. Comm., 2012, 14(3), 1111-1116.
- C. L. Henry, C. N. Dalton, L. Scruton, V.S.J Craig, Ion-specific coalescence of bubbles in mixed electrolyte solutions, J. Phys. Chem., 2007, (111), 1015-1023.
- J. Toth, A. Kardos-Fodor, S. Halász-Péterfi, The formation of fine particles by salting-out precipitation, Chem. Eng. Process.: Process Intensification, 2005, 44(2), 193-200.
- B. W. Ninham, R. M. Pashley, P. Lo Nostro, Surface forces: Changing concepts and complexity with dissolved gas, bubbles, salt and heat, Curr. Opin. Colloid Interface Sci., 2016, 27, 25-32.
- M. Taseidifar, J. Anthony, R.M. Pashley, Prevention of fluid cavitation, In press: Substantia: An International Journal of the History of Chemistry, 2020.
- H. K. Kim, E. Tuite, B. Norden, B.W. Ninham, Co-iondependence of DNA nuclease activity suggests hydrophobic cavitation as a potential source of activation energy Eur. Phys. J. E, 2001, 4, 411–417.
- S. V. Gudkov, G. A. Lyakhov, V.I. Pustovoy, I.A. Shcherbakov, Influence of Mechanical Effects on the Hydrogen Peroxide Concentration in Aqueous Solutions, Phys Wave Phenom, 2019, 27(2), 141-144.
- B. W. Ninham, P. Lo Nostro, Unexpected Properties of Degassed Solutions, J. Phy. Chem. B, 2020, 124(36), 7872-7878.
- A. E. Voinescu, D. Touraud, A. Lecker, A. Pfitzner, W. Kunz, B. W. Ninham, Mineralization of CaCO3 in the Presence of Egg White Lysozyme, Langmuir, 2007, 23, 12269-12274.
- B. P. Reines, B.W. Ninham, Structure and function of the endothelial surface layer: unraveling the nanoarchitecture of biological surfaces, Quarterly Rev. Biophys., 2019, 52, 1–11.
- A. G. Sanchis, R. M. Pashley, B. W. Ninham, Virus and bacteria inactivation by CO2 bubbles in solution, NPJ Clean Water, 2019, (2) Number 1.
- A. G. Sanchis, L. Jin, Evaluation of the new energy-efficient hot bubble pilot plant (HBPP) for water sterilization from the livestock farming industry, Water Resour. Ind., 2020, 24, 100135.




